TGA Australia promotes Lethal IgG4 Keytruda Pembrolizumab
Thousands of people have died, including 205 in Australia.
I received an email update from Annals of Internal Medicine including a case report1 from Taiwan of a 44-year-old woman who died of Cytokine release syndrome after treatment with Keytruda, also known as Pembrolizumab (formerly Lambrolizumab).
In this case massive increase in IL-62 was observed before her death.
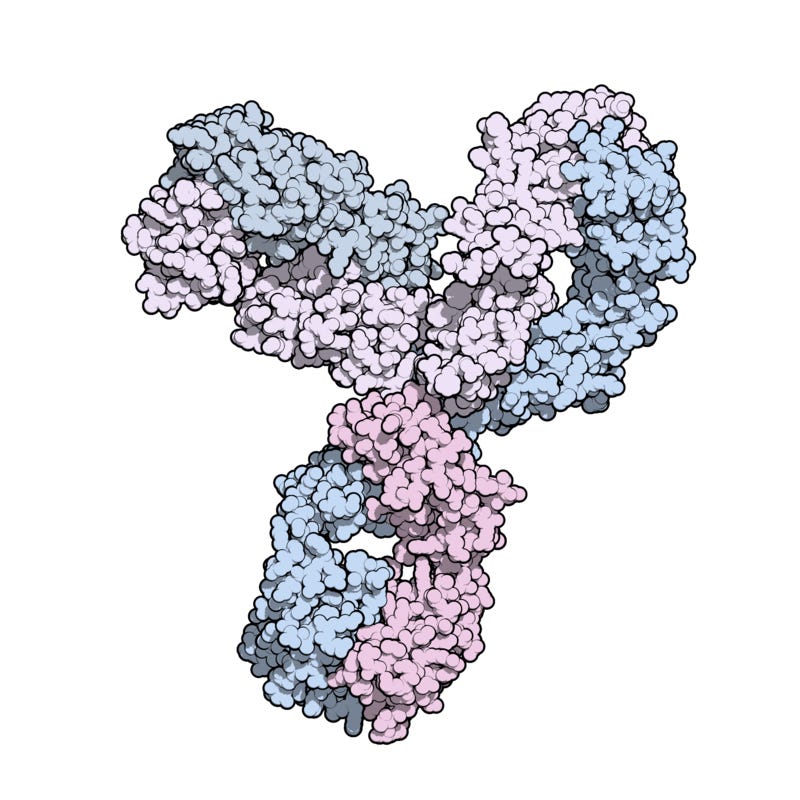
David Cowley has mentioned the huge Adverse Reaction and Withdrawal from use of Keytruda (Pembrolizumab) which is a humanized monoclonal antibody, specifically an IgG4 isotype, that acts as a PD-1 inhibitor, a type of cancer immunotherapy, used to treat various cancers by blocking a mechanism that allows cancer cells to evade the immune system and the Endotoxin problem in a post from May 2024.3
Picture credit.4
More about IgG4 here.5
Keytruda molecular weight is 146648.64 g·mol−1 , formula is C6534H10004N1716O2036S46
Subscribers will note the similarity of the molecule to Omalizumab.6
Wikipedia gives a detailed history of the marketing of this drug.7
So I visited US FAERS and found 9,749 Deaths, 40,690 Serious Adverse Event reports from 49,638 case reports.
Look at the exploding case numbers for Keytruda at FAERS.
TGA actively promoting Keytruda
Then I visited the TGA DAEN and found 205 Deaths in Australia.
Then I searched for more information and found TGA has been expanding approvals for use of this killer drug to different types of Cancer treatment attempts in “Project Orbis”, collaborating with the US FDA and Health Canada.
TGA provided updated information on Early Deaths followed by “overall survival benefit” seen in clinical trials in August 2022.
As reported in numerous clinical trials, the claimed “overall survival benefit” was only a matter of months.
TGA was obviously aware that Adverse Event are under-reported, so chose this page to encourage “suspected” adverse reactions.8
Cytokine Release Syndrome (CRS)
PubMed lists 7,087 peer reviewed papers on CRS. Of those 315 mention Endotoxin, 369 mention Lipopolysaccharide and 303 mention LPS.
PubMed is apparently programmed to find CRS synonyms9 including Cytokine Storm Syndrome (CSS), Systemic Inflammatory Response10 Syndrome (SIRS), etc.
The key is the Positive Feedback Loop of Inflammation that I have mentioned numerous times that defeats the mythical mantra that “The Dose Makes the Poison”.
Endotoxin in Keytruda
Searching TGA yields Zero documents mentioning the obvious supertoxin that will be present and vary batch to batch because Keytruda is made in Chinese Hamster Ovary cells (CHO). Some patients receive 400 milligram (mg) of the drug as a single intravenous dose.
A study from Iran11 measured Endotoxin in Keytruda at 0.5 ± 0.2 EU/mg so we can be confident that patients receive a massive Endotoxin hit with every treatment, way above any dose given in Human volunteer jabbing studies and about 30 times as much as found in a Pfizer jab.1213
FDA Approval in 2014 with No Endotoxin Data
As we expect from the US Bioweapons developers at Silver Spring Maryland14, Keytruda was given approval by US FDA in 2014 with the proviso15 that Merck Sharpe and Dohme do some experiments by spiking samples with large amounts of Endotoxin to estimate the anticipated Low Endotoxin Recovery using the Horseshoe Crab LAL test.
LICENSING
We have approved your BLA for Keytruda (pembrolizumab) effective this date. You are hereby authorized to introduce or deliver for introduction into interstate commerce, Keytruda under your existing Department of Health and Human Services U.S. License No. 0002. Keytruda is indicated for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor.
MANUFACTURING LOCATIONS
Under this license, you are approved to manufacture Keytruda drug substance at MedImmune LLC, Frederick Manufacturing Center in Frederick, Maryland.
The drug product will be manufactured and filled at Schering Plough Brinny Co. in Cork, Ireland.
The final formulated product will be labeled, and packaged at Merck Sharp & Dohme Corp. in Wilson, North Carolina.
You may label your product with the proprietary name Keytruda and will market it in 50 mg single-use vials.
Keytruda is another example where the adverse events can’t be ascribed to LNPs or mRNA, although Host cell and Plasmid DNA residue is likely.
Surge in IL-6 after electroporation of Plasmid DNA can be attributed to Endotoxin because typical levels used in experiments were found to be huge <0.1 EU/μg or <100 EU/milligram.16
Moderna mRNA used with Keytruda
Mind-boggling news shared by a number of authors was the announcement in 2022 that suffering cancer patients would be used in a trial of an mRNA jab combined with Keytruda.17
Other Endotoxin induced Harms
Approaching the email length limit, but can add more references later.
One that caught my eye was Stevens-Johnson Syndrome in a 75-year-old lady 14 days after being hit with Keytruda.18 I have an article on this nasty disease.19
Time to Withdraw Keytruda
Endotoxin is the key and all batch analysis measurements should be released to assist with legal action before the drug is withdrawn.
June 2025 Update
Mark Aldred asked AI about the long term effects of shifting your immune system to IgG4 tolerance.20
Wei Hung Hung, Yu-Sheng Hu, Li-Hua Fang and Rong-Long Chen. 2025. Fatal Outcome of Pembrolizumab-Related Cytokine Release Syndrome in a Patient With Concurrent Parainfluenza Infection. AIM Clinical Cases. 4:e231217. doi:10.7326/aimcc.2023.1217
Drew Weissman Endotoxin induced Interleukin-6 Legacy
In my recent article I pointed to a new patent application by Drew Weissman and colleagues in which it was revealed that mRNA LNP jabs massively increased Endotoxin Interleukin-6 damage and death.
Fvasconcellos - From PDB entry 5DK3. More information: Scapin G, Yang X, Prosise WW, et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol 2015;22(12):953-8. doi:10.1038/nsmb.3129
IgG4 affected by Endotoxin in mRNA Jabs
There has been lots of discussion about IgG4 here on Substack and elsewhere recently, increasing with each mRNA Covid19 Jab.
Omalizumab Xolair Death Toll is staggering
Omalizumab, trade name Xolair is a drug for the treatment of moderate to severe persistent asthma in adults and adolescents. It is an enormous molecule C6450H9916N1714O2023S38 with Molecular Weight 145,058.53 gram·mol−1
https://en.wikipedia.org/wiki/Pembrolizumab
https://www.tga.gov.au/news/safety-updates/product-information-safety-updates-september-and-october-2022
https://en.wikipedia.org/wiki/Cytokine_release_syndrome
Kier Starmer appoints Endotoxin Expert Patrick Vallance as Science Minister within Hours of Election
They both have knighthoods, so should I address them as Sir?
Morteza Jaffar-Aghaei, Farzad Khanipour, Amir Maghsoudi, Rahim Sarvestani, Mahdi Mohammadian, Maryam Maleki, Forugh Havasi, Hossein Rahmani, Amir-Hossein Karagah, Mohammad-Reza Kazemali. 2022. QbD-guided pharmaceutical development of Pembrolizumab biosimilar candidate PSG-024 propelled to industry meeting primary requirements of comparability to Keytruda. European Journal of Pharmaceutical Sciences 173:106171
Fauci the Endotoxin expert studied Individual Jab Susceptibility from 1974
I was talking to my friend a couple of days ago about the value of tracing research outputs of individuals involved in mass Jabbing and their detailed knowledge of Endotoxin Harms.
Measuring Endotoxin in Jabs with a Fluorescent Reaction
Here is a short article on the ThermoFisher Invitrogen Qubit Flex Fluorometer.
Centreville High School students worked with the Virus Leaking Lab at Silver Spring
Please see Part 4 and preceding posts on this Military Bioweapon Community.
Food and Drug Administration Silver Spring MD 20993. 14 September 2014. CDER. BLA 125514/0. BLA ACCELERATED APPROVAL.
Loree C. Heller, Guilan Shi, Amanda Sales Conniff, Julie Singh, Samantha Mannarino, Jody Synowiec and Richard Heller. 2024. IL-12 and PD-1 peptide combination gene therapy for the treatment of melanoma. Molecular Therapy: Nucleic Acids 35 https://doi.org/10.1016/j.omtn.2024.102267
Moderna and Merck Announce mRNA-4157/V940, an Investigational Personalized mRNA Cancer Vaccine, in Combination with KEYTRUDA(R) (pembrolizumab), Met Primary Efficacy Endpoint in Phase 2b KEYNOTE-942 Trial. https://investors.modernatx.com/news/news-details/2022/Moderna-and-Merck-Announce-mRNA-4157V940-an-Investigational-Personalized-mRNA-Cancer-Vaccine-in-Combination-with-KEYTRUDAR-pembrolizumab-Met-Primary-Efficacy-Endpoint-in-Phase-2b-KEYNOTE-942-Trial/default.aspx
Michael Sandhu, Binod KC, Jenish Bhandari, Harvir S Gambhir, Ramsay Farah. 2023. Pembrolizumab-Associated Stevens-Johnson Syndrome in a Patient With Metastatic Non-small Cell Lung Cancer: A Case Report. https://www.cureus.com/articles/166825-pembrolizumab-associated-stevens-johnson-syndrome-in-a-patient-with-metastatic-non-small-cell-lung-cancer-a-case-report#!/
Stevens-Johnson Syndrome caused by Endotoxin in Jabs
Recently I asked readers to name a disease that can’t be linked to Endotoxin in Covid19 jabs, and one suggested Tinea (but see below). So I have been quietly gathering material on damage to our largest organ, namely Skin, by the Covid19 jabs.