TGA posts Misinformation on its DNA and Endotoxin testing of Covid19 Jabs
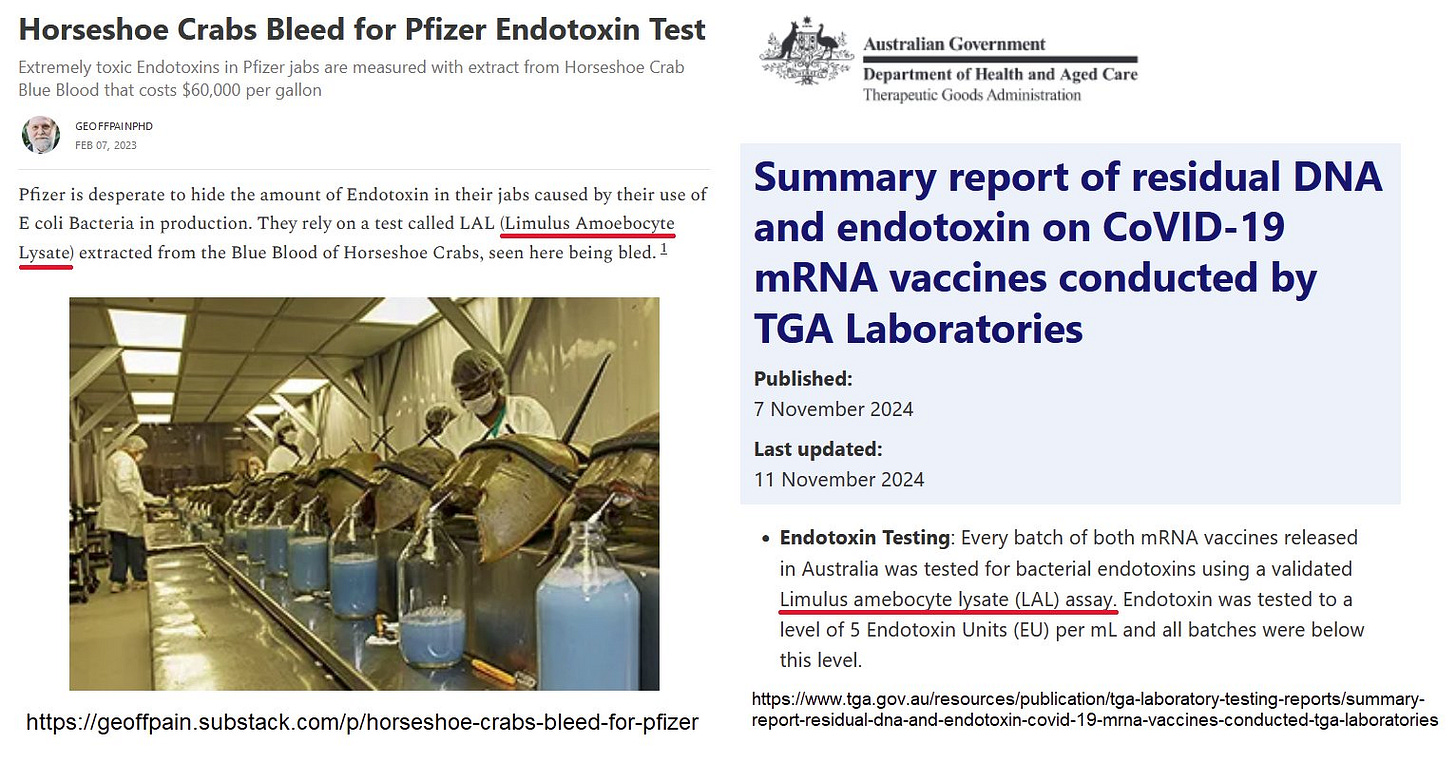
After 4 years refusing to report any measurements of DNA, which they commenced in 2021, Therapeutic Goods Administration published a "Summary Report". Let's look at Endotoxin etc.
Thanks to Rebekah Barnett for letting me know that the report1 was posted on 7 November 2024 and edited on 11 November.
Thanks to Chuck for announcing it on X2 and reminding us the TGA still exploits Horseshoe Crabs by buying its hydrolyzed Blue Blood.3
I was amazed that the title contains two whopping errors. TGA has invented “CoVID-19” and uses “on” instead of “in”.
TGA Endotoxin and DNA Covid19 Jab testing from 2021
We know from Freedom of Information (FOI) documents that TGA tested many lots in its Canberra lab. Here is an example of Pfizer Lot FL7649.
TGA used Pfizer supplied Drug Substance mRNA Lot 21Y513C6101 as reference.
Another FOI document provides extra information.
Note that the Endotoxin Limit is redacted and no measurement is shown.
Pyrosperse Use Not Justified
We see that TGA used Pyrosperse detergent, that points to using a Lonza LAL test kit. It is a Calcium modified polyanionic mixture with undisclosed exact composition, marketed by Lonza for use with its PYROGENT™, QCL-1000™ and Kinetic-QCL™ Limulus Amebocyte Lysate (LAL) Assays.4
In FOI 4878 the TGA includes a statement that does not inspire confidence:
Pyrosperse™ Dispersing Agent (used in conjunction with KQCL kit - specifically validated products only)
Is intended for use with the KQCL assays to assist in the qualitative or quantitative detection of bacterial endotoxin. Pyrosperse™ is one of a group of metallo-modified polyanionic dispersants which has proven useful as a sample modifying agent for certain types of products showing inhibition in the LAL assay. In those products for which endotoxin binding is the suspected source of inhibition, the use of Pyrosperse™ should be considered. To date, Pyrosperse™ has been found useful in LAL endotoxin detection when used with the following products: Human Serum Albumin, 5% and 25%; Plasma Protein Fraction; Electrolyte solutions; Antihemophilic Factor; and Lipid emulsions.
Additional product applications may exist.
TGA does not provide any proof that the Lonza Kit works with LNPs.
TGA Trapped in Erroneous Assumption Logic Loop
There are different types of scientists, those who think, and those who are comfortable using Standard Operating Procedures (SOPS).
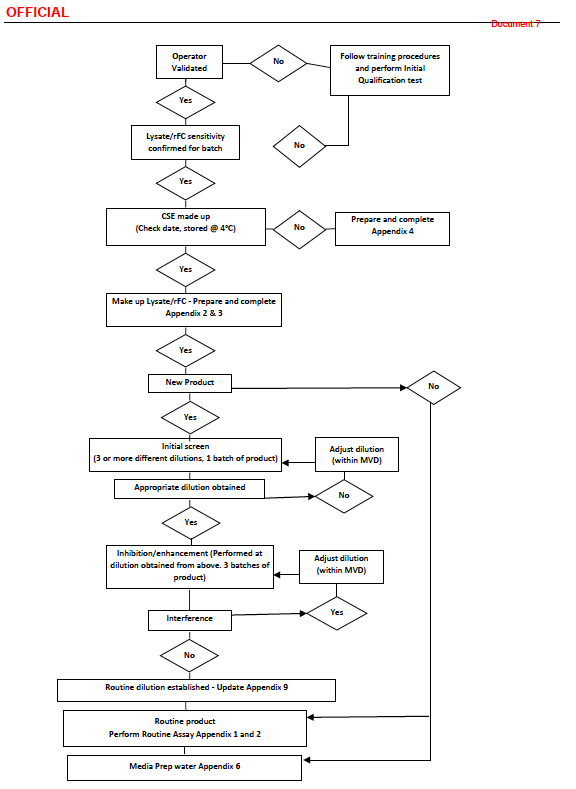
Here we see the TGA Flowchart that sinks them.
Observe the ludicrous diamonds at top right that end in “No” with nowhere to go!
In the middle, see rectangle “New Product” - as in Covid19 Jabs with chemical and physical entrapment of Endotoxin.5
They make the Erroneous Assumption that Dilution will solve all their problems.
Then they dilute the sample repeatedly and use that as a excuse to report a False “Limit of Detection” of <5 EU/ml.
In her recent article, my friend Maryanne Demasi6 said, apparently swallowing the TGA line:
NB: All batches were also tested for bacterial endotoxin content by the TGA Labs and found to comply with the allowed limits.
My readers know full well that the TGA claim that injecting 350 EU of Endotoxin into a 70 kilogram person is “safe” is a complete Lie, given that Human volunteers suffer immediate damage at levels of less than 1 nanogram per kilogram of bodyweight7 including breach of the Blood Brain Barrier8 and we know exactly why Women suffer more than Men.9
Lots of people are still asking questions about Endotoxin measurement in jabs.10
As this went beyond email length limits, please see the following article on problems caused by the LNPs with more references to problems caused by LAL interfering jab ingredients.11
https://www.tga.gov.au/resources/publication/tga-laboratory-testing-reports/summary-report-residual-dna-and-endotoxin-covid-19-mrna-vaccines-conducted-tga-laboratories
https://x.com/ChuckC_0423/status/1856323887602307076
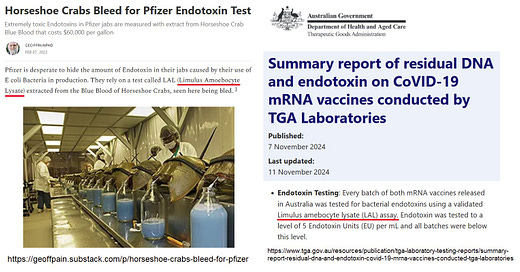
Horseshoe Crabs Bleed for Pfizer Endotoxin Test
Pfizer is desperate to hide the amount of Endotoxin in their jabs caused by their use of E coli Bacteria in production. They rely on a test called LAL (Limulus Amoebocyte Lysate) extracted from the Blue Blood of Horseshoe Crabs, seen here being bled.
Lipid Nanoparticles, Endotoxin and Detergent Micelles
We know that Covid19 Jab manufacturers are desperate to stop Jabbees learning how much Endotoxin is in the various Batches and Brands of the toxic soups.
Moderna ordered to get its Endotoxin Act together
Despite heavy redactions, including specifications and contamination limits, we learn much from reading the thousands of pages obtained by citizens in Freedom Of Information demands as well as court ordered releases.
German Expertise in Jab Endotoxin Pain
In an earlier article I reported on the Pain reported by Covid19 Jabbees and proposed mechanisms for short-term Pain progressing to Constant Chronic Pain.
Fatigue, Headache, Muscle Pain, Fever and Chills after Pfizer Jabs match Endotoxin Effects
Billions have been told Jab Symptoms are “Normal”
Paul Ehrlich Institute Proves Endotoxin in Jabs Harms Women more than Men
Numerous people, including me, have written about the fact that Women of all ages are hit harder than men by the crazy Covid19 Jabs.
How did I get on their Endotoxin Mailing List?
Nice to see such activity by the United States Pharmacopeial Convention.
Busting open the LNPs to get to the Endotoxin
A wide range of Jabs exhibit Low Endotoxin Recovery (LER) in attempts to measure the supertoxin using the Limulus Amebocyte Lysate (LAL) test developed by my distant cousin.