Lipid Nanoparticles, Endotoxin and Detergent Micelles
Endotoxin content of Covid19 Jabs is difficult to measure, so let's look at some vital Chemistry that can help expose the truth.
We know that Covid19 Jab manufacturers are desperate to stop Jabbees learning how much Endotoxin is in the various Batches and Brands of the toxic soups.
In earlier articles I mentioned the problems with Endotoxin measurements in AstraZeneca1, Janssen, Moderna and Pfizer jabs2 and attempts to abandon the Horseshoe Crab LAL tests in favour of Mass Spectrometry or Optoelectronic Sensors.3
Detergents are used in the hope that they will break up micelle aggregates of Endotoxin.
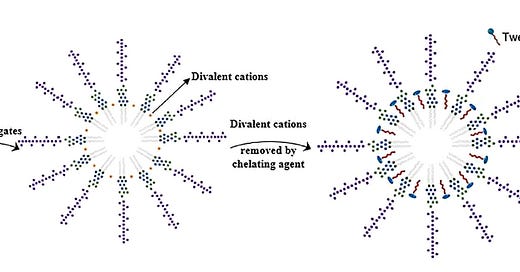
Endotoxin is composed of variable sized chunks of Bacterial Cell Walls and typically exists as Micelles in water. This Figure from Cao and coworkers4, who discuss the impact of Micelles on attempts to measure the toxin, illustrates the effect of a common high molecular weight detergent, Tween 20 (Polysorbate 205), on Endotoxin Micelles in the presence of Divalent metal Cations, which might be removed with Chelating agents such as EDTA, Citric Acid or residues of other jab contaminants like HEPES.
Triton X-100 (2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol, Polyethylene glycol p-(1,1,3,3-tetramethylbutyl)-phenyl ether)6 has also been found to interfere with the LAL test.7
Recently I learned that the Therapeutic Goods Administration uses a detergent in its Endotoxin measurements employing Horseshoe Crab Blood products or cloned derivatives of clotting factor enzymes.
The PYROSPERSE™ Dispersing Agent, or detergent, is a Calcium modified polyanionic mixture with undisclosed exact composition, marketed by Lonza for use with its PYROGENT™, QCL-1000™ and Kinetic-QCL™ Limulus Amebocyte Lysate (LAL) Assays.
As early as 1966 scientists involved in the US Endotoxin Reference Supply, Rudbach and Milner8 reported effects of detergents when studying the enzymes involved in LAL and their need to contact the Lipid A portion, which is the preferred “Adjvant” in the Pfizer jab.9
It has been said Calcium is essential for the LAL test and I will expand on that claim later.
Lauryl Sulfonate Salts are Strongly Alkaline
Many household products use the Sodium salt of the very strong acid Dodecyl Sulfonic Acid (also known as Sodium Lauryl Sulfonate, SLS or SDS).10 When looking for useful information I found a nice introductory article to the complicated science at the University of Bristol Chemistry department where I worked from 1979 to 1981.11
The Lithium salt is preferred for some applications and it commonly yields a pH up to 9 unless very dilute, which is out of the range of many laboratory tests.12
Lauryl Sulfonate forms its own LNPs
Above a critical concentration of 2.34 gram per Litre, SDS will form micelles in water. These contain 20 to 50 molecules of the anion.13
Turbidity Interferes with LAL tests
Analysts frequently have no idea of what Endotoxin level will be in a particular sample and can encounter interfering turbidity. They usually try multiple dilution to reduce this effect, as reported by Lonza in 2015.14
SDS easily forms insoluble salts with cationic organics, which might account for its effectiveness at low concentrations in breaking up micelles formed with Cationic Lipids like ALC-0315, [(4-hydroxybutyl)azanediyl]di(hexane-6,1- diyl) bis(2-hexyldecanoate) used in Pfizer jabs and its impurities.15
Disrupting LNPs with other Neutral Polar Organics
I was suggesting to my friend the other day that Methylene Chloride (Dichloromethane) might be useful in our study of how much Endotoxin is riding outside, inside and possibly built into the LNP walls.16 It was one of my favourite solvents in my organometallic chemistry and later natural products chemistry experiments.
It is somewhat safer than Chloroform (Trichloromethane), but still requires use of a good fumehood.
I found an interesting paper from Poland where Methylene Chloride was used to break open LNPs.17
That was after I found a paper from Britain and Denmark that looked at alcohols used in formation of LNPs and Liposomes.18 And another interesting paper on Niosomes.19
My friend just found a paper on use of Dimethyl Sulfoxide used to make some drug formulations give better LAL testing.20
What is the effect of Vortexing the Jab Sample?
Because we are interested in measuring where most of the Endotoxin resides, different treatments such as vortexing or centrifugation could be applied as variables.
Real Endotoxin Measurements are in
Patience and thoroughness are the marks of a brilliant Endotoxin LNP Jab analyst, so I was excited by the announcement of Kevin McKernan’s Endotoxin results.
Measuring Endotoxin in Jabs with a Fluorescent Reaction
Here is a short article on the ThermoFisher Invitrogen Qubit Flex Fluorometer.
Yuan Cao, Yujie Zhang, Frank Qiu. 2021. Low endotoxin recovery and its impact on endotoxin detection. https://onlinelibrary.wiley.com/doi/abs/10.1002/bip.23470
https://en.wikipedia.org/wiki/Polysorbate_20
https://en.wikipedia.org/wiki/Triton_X-100
Takanori Nakamura, Fuminori Tokunaga, Takashi Morita, and Sadaaki Iwanaga. 1988. Interaction between Lipopolysaccharide and Intracellular Serine Protease Zymogen, Factor C, from Horseshoe Crab (Tachypleus tridentatus) Hemocytes. https://academic.oup.com/jb/article-abstract/103/2/370/1122824
Jon A. Rudbach and Kelsey C. Milner. 1968. Canadian Journal of Microbiology. Reaction of endotoxin and surfactants. III. Effect of sodium lauryl sulfate on the structure and pyrogenicity of endotoxin. https://cdnsciencepub.com/doi/10.1139/m68-197
https://en.wikipedia.org/wiki/Sodium_dodecyl_sulfate
Zara Kauffer and Paul May. 2010. Molecule of the Month March 2010. Sodium lauryl sulfate. The main cleaning agent in soap and detergent. https://www.chm.bris.ac.uk/motm/SLS/SLSh.htm
https://www.fengchengroup.com/chemicals/mineral-inorganic-substance/lithium-dodecyl-sulfate-lds-cas-2044-56-6.html
Nicholas J Turro and Ahmad Yekta. 1978. Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. https://pubs.acs.org/doi/pdf/10.1021/ja00486a062
Ariane C.S. Marinho, Ana R.O. Polay, and Brenda P.F.A. Gomes. Accuracy of Turbidimetric Limulus Amebocyte Lysate Assay for the Recovery of Endotoxin Interacted with Commonly Used Antimicrobial Agents of Endodontic Therapy. https://www.jendodon.com/article/S0099-2399(15)00506-3/fulltext
Zhichang Yang, Paul Baker, Sahana Mollah, Robert Proos and Jonathan Le Huray
SCIEX, USA; Acuitas Therapeutics Inc. Structural characterization of the cationic lipid nanoparticle component, ALC-0315, and its impurities using electronactivated dissociation (EAD)-based MS/MS fragmentation. https://sciex.com/tech-notes/biopharma/structural-characterization-of-the-cationic-lipid-nanoparticle-c
Eliza Wolska and Marta Brach. Distribution of Drug Substances in Solid Lipid Microparticles (SLM)—Methods of Analysis and Interpretation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8879661/
Cameron Webb, Swapnil Khadke, Signe Tandrup Schmidt, Carla B. Roces, Neil Forbes, Gillian Berrie, and Yvonne Perrie. 2019. The Impact of Solvent Selection: Strategies to Guide the Manufacturing of Liposomes Using Microfluidics. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6955969/
Mohammad A Obeid, Saja Haifawi and Ibrahim Khadra. 2023. The impact of solvent selection on the characteristics of niosome nanoparticles prepared by microfluidic mixing. https://www.sciencedirect.com/science/article/pii/S2590156723000129
Christopher W. Lester, J. Timothy Ramsey and Jonathon S. Salsbury. 2021. Bacterial Endotoxins Testing of Water-Insoluble Drug Substances by Means of Dispersion with Dimethyl Sulfoxide. https://www.americanpharmaceuticalreview.com/Featured-Articles/574840-Bacterial-Endotoxins-Testing-of-Water-Insoluble-Drug-Substances-by-Means-of-Dispersion-with-Dimethyl-Sulfoxide/