AstraZeneca Endotoxin Test Meaningless
EDTA in AstraZeneca jabs is capable of inhibiting the Horseshoe Crab Blood test resulting in completely invalid test results. No wonder they won't publish results!
In a previous articles I have shown that AstraZeneca was withdrawn because it was killing a higher proportion of people that Pfizer or Moderna1 and causing Eye Damage.2
Delving further, I now find that the complete conspiracy of suppression of AstraZeneca Endotoxin measurements is probably hiding embarrassing deadly facts.
In this scheme we see the Cytokine Storm summary for Adenovirus jabs.3
Note that all of the Cytokines and Chemokines are upregulated by Endotoxin alone.
RANTES = CCL5 named by Dr. Tom Schall Argentine movie alien who shows up in a mental ward who was named Rantés.4
RANTES top interactions creating disease are shown at the CTD.5
AstraZeneca Endotoxin contamination measurements - Please Help gather the data
Calling all those who have collections of papers on the Chimpanzee Adenovirus AstraZeneca jab production and quality control measurements. I would like to know Endotoxin levels in the genetically modified Human Embryonic Kidney (HEK) 293 cells and in all additives in the brew.
In addition to the Chimpanzee AdenoVirus, the AstraZeneca jabs contain:
0.1mM EDTA 10mM Histidine 7.5% Sugar 0.5% Ethanol and 0.1% Polysorbate 80.
I have covered toxicity of these ingredients already.6789
EDTA inhibits Horseshoe Crab Blood tests
Endotoxin tests employed by regulatory authorities rely on the Horseshoe Crab Blood test that is covered in an earlier article.10
Now I find literature from the 1970s demonstrating that EDTA is capable of reducing or even eliminating sensitivity to Endotoxin.11
Japanese researchers showed that EDTA lowers the pH of the test solution lowering the sensitivity further, or under certain conditions elminating it all together, in millimolar concentrations.12
Pfizer Endotoxin Tests are not reliable either
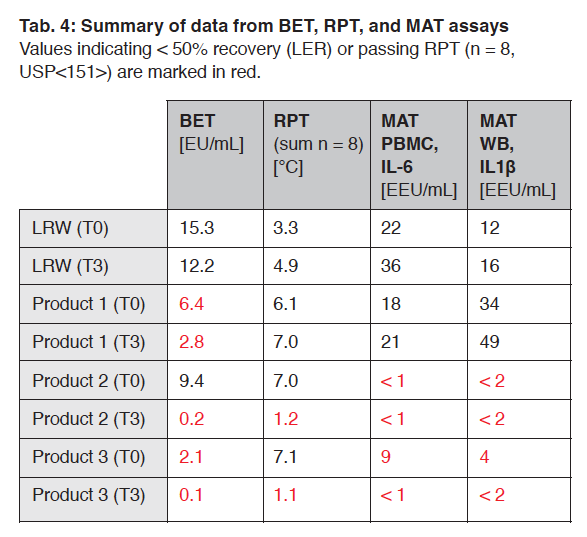
Just found a 2023 publication by Pfizer and some other manufacturers comparing Endotoxin test methods, showing results are made invalid not only by other compounds in the mix, but changes in as little as 3 days after manufacture.13
They studied 3 unnamed products containing:
Product 1 Histidine, Trehalose, EDTA, and Polysorbate 80
Product 2 Citrate, Lysine Monohydrate, Trehalose, and Polysorbate 80
Product 3 Charged, Aliphatic Amino Acid and Surfactant
Trehalose looks like this:
In their comparison Table 4, note the wild variation in results from the nominal 19.7 EU/ml expected for the reference standard spiked samples. The Reference Standard Endotoxin was (Cat#E700 (USP) at 10,000 EU/vial) was reconstituted with 1 mL of LAL reagent water (LRW, EndoSafe®) to reach a huge concentration of 10,000 EU/mL, then diluted.
BET = the kinetic chromogenic Limulus Amebocyte Lysate (LAL) test, according to current USP<85> and Ph. Eur. 2.6.14 (EDQM, 2020b)
RPT = Rabbit Pyrogen Test performed using a hybrid breed, a cross between a European domestic rabbit (Deutsches Hauskaninchen) and New Zealand Whites. A total of 64 rabbits were used with body weights ranging from 1.6 to 2.3 kg.
MAT PBMC = Monocyte Activation Test on Human Peripheral Blood Mononuclear Cells using Sanquin reagents (M2016 MAT Cell Set, pMAT cells (cryopreserved, pooled from 4 donors), and MAT culture medium supplements, M1916 Pelikine Compact Human IL-6 kit and M1980 Pelikine Tool Set from Sanquin, Amsterdam, The Netherlands
MAT WB = Monocyte Activation Test on Whole Blood using Merck KGaA reagents (1.44155.0001: PyroDetect Cryoblood (cryopreserved human WB from 8 donors), KHC0011: IL-1 Beta Human ELISA Kit, E0150000: Pyro-Detect Endotoxin Standard, Iscove’s Modified Dulbecco’s medium (IMDM) with L-glutamine and 25mM HEPES).
LRW = LAL-Reagent Water from Lonza
Note that HEPES is known to interfere with the LAL test.
TGA allowed AstraZeneca use without adequeste Endotoxin Measurements
Buried at the bottom of a TGA webpage14, we find under “Post approval quality commitments”
The additional data related to validation of analytical procedures for endotoxin testing should be provided.
Has any Senator mamanged to find out whether this was done?
Relative Lethality of COVID-19 vaccines - who is measuring the casualties?
Japan Relative Lethality data for the Covid19 Jabs This is an update to the earlier version of this article. Useful data on Total Deaths and Myocarditis Deaths after jabs by brand from Japan. Number of first and second dose by vaccine type until 14 February 2022 - Deaths - Deaths per million jabs and Myocarditis Deaths per million jabs with data to 11 Aug…
Blindness from AstraZeneca Jabs
I was given, by an Australian doctor, comparative data from the UK Government Yellow Cards Adverse Events Database, showing that there were more symptoms of Eye Disorder for AstraZeneca jabs compared to Pfizer or Moderna Covid19 jabs as a proportion of total reports.
Ahi YS et al. 2011. Adenoviral Vector Immunity: Its Implications and circumvention strategies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4009923/
https://en.wikipedia.org/wiki/CCL5
https://ctdbase.org/detail.go?type=gene&acc=6352
EDTA in AstraZeneca Kills via VITT
In previous articles I have called out the Fraudsters who seek to deliver EDTA into the veins of their Dupes by convincing them that there is Graphene Oxide in mRNA jabs and that multiple doses of EDTA will make jabbees miraculously better. AstraZeneca contains the Killer ingredient EDTA
Acetaldehyde from Ethanol in Pfizer jabs causing Batch Lethality variation?
As I and others have highlighted, the mRNA jabs are inherently dangerous due to the GMO mRNA and the other ingredients including Sugar and the 4 chemicals used to make the Lipid Nanoparticles (LNPs) and the Endotoxin from E Coli. During manufacture, the 4 precursors of LNPs are dissolved in Ethanol and the mRNA is dissolved in acidified water. Then the 2…
Polysorbate 80 in Covid19 Jabs a Major Hazard
I was pleased to find a major thread on Twitter from 2021, where I discussed the hazards of Polysorbate 80 in Covid19 jabs, remains intact after Elon Musk restored my account. Polysorbate is used in AstraZeneca and Janssen jabs. So I can build on that with updates, meanwhile sharing early.
Sugar is the Major Ingredient of mRNA Jabs
All of the ingredients, or “excipients”, in the mRNA jabs have been declared and there are no “secret” proprietary chemicals added. However there is rampant mythology. Recently I was told "at least 26 researchers/research teams in 16 countries, using various microscopic methods of analysis, have reported the presence of undeclared microscopic geometric a…
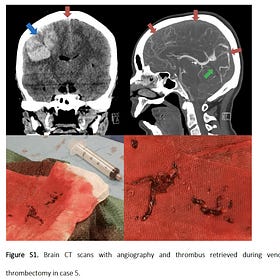
Horseshoe Crabs Bleed for Pfizer Endotoxin Test
Pfizer is desperate to hide the amount of Endotoxin in their jabs caused by their use of E coli Bacteria in production. They rely on a test called LAL (Limulus Amoebocyte Lysate) extracted from the Blue Blood of Horseshoe Crabs, seen here being bled.
Kenney DM, et al. 1972. AGGREGATION OF HORSESHOE CRAB (LIMULUS POLYPHEMUS) AMEBOCYTES AND REVERSIBLE INHIBITION OF AGGREGATION BY EDTA. https://www.journals.uchicago.edu/doi/10.2307/1540183
Kobayashi M and Yamamoto M. 1975. Limulus Lysate(Pre-gel)のゲル化反応に関する研究(第4報)アルカリ土類金属およびキレート剤のゲル化反応に及ぼす影響 https://www.jstage.jst.go.jp/article/yakushi1947/95/8/95_8_959/_article/-char/ja/
Thurman TL, Lahti CJ, Mateffy JM, Forng R-Y, von Wintzingerode F, Silva LM, Deutschmann SM, Mozier NM. 2023. Comparison of pyrogen assays by testing products exhibiting low endotoxin recovery. https://www.altex.org/index.php/altex/article/view/2432
https://www.tga.gov.au/resources/auspmd/covid-19-vaccine-astrazeneca