FDA detected Endotoxin by Mass Spectrometry
The US FDA accepts Horseshoe Blood Limulus Amoebocyte Lysate (LAL) tests for Endotoxin in lieu of the Rabbit Pyrogens Test. Why don't they use reliable tests?
As mentioned in earlier posts Gram Negative Bacterial Cell fragments known as Endotoxin and its supertoxin Lipid A are deliberately1 incorporated into the Pfizer Covid19 and Novavax jabs and are also present in AstraZeneca, Janssen, Moderna jabs because no technology was applied to remove them.
Endotoxin varies with species of Bacteria
A nice cryoelectron tomography image of a single E. coli bacterial cell showing the location of the toxin was published in 2019.2
Note the Inner Membrane (IM) and Outer Membrane (OM) (scale bar 200 nm), PeptidoGlycan (PG), LipoPolySaccharide (LPS), anchored to the membrane by the LPS Lipid A which consists of a β-1′,6-linked disaccharide of Glucosamine that is both phosphorylated and fatty acylated. The inner core (Kdo (3-deoxy-D-manno-octulosonic acid). (d) shows LPS modifications that occur in Salmonella.
Ingredients in the jabs can hide or mask the actual Endotoxin levels, as mentioned with EDTA and Polysorbate 80 in the case of AstraZeneca.
AstraZeneca Endotoxin Test Meaningless
In a previous article I have shown that AstraZeneca was withdrawn because it was killing a higher proportion of people that Pfizer or Moderna. Delving further, I now find that the complete conspiracy of suppression of AstraZeneca Endotoxin measurements is probably hiding embarrassing deadly facts.
Here I expand on the False low Endotoxin measurements, which will have Legal implications because accurate reliable tests are available to quantify the toxic load.3
Crazy Variation in FDA Endotoxin Limits
Before getting into details of why you can’t trust any limits imposed on medical “devices” which includes jabs, here are a few limits in Endotoxin Units that have been found, largely not updated for decades and all based on the Horseshoe Crab LAL test.
Products that directly or indirectly contact the Cardiovascular system and Lymphatic system.
0.5 EU/mL or 20 EU/device
Devices in contact with Cerebrospinal fluid (e.g. Epidural), the limit is
0.06 EU/mL or 2.15 EU/device.
There is evidence that the FDA accepted an arbitrary limit of 5 EU/ml for Covid19 jabs and the European Union allowed 12.5 EU/ml.
FDA Mass Spectrometry Endotoxin Measurement
I used Mass Spectrometry4 to help identify new molecules I made starting in 1974.
In 1990 researchers in Sweden pointed to the inadequacy of the LAL test and reported a faster, more sensitive method based on gas chromatography and Mass Spectrometry.5
In 2004, chemical derivatization of the Lipid A fraction of Endotoxin was used to detect as little as 7 picogram per sample using Gas Chromatography combined with Mass Spectrometry.6
In 2012 Mass Spectrometry was used to identify a strain of Shigella that causes dysentery responsible annually for one million fatalities mostly among infants.7
In 2015 researchers in France8 proved that Mass Spectrometry could be used to detect Endotoxin where LAL tests failed.
The Eyes are especially sensitive to Endotoxin as mentioned in an earlier article.
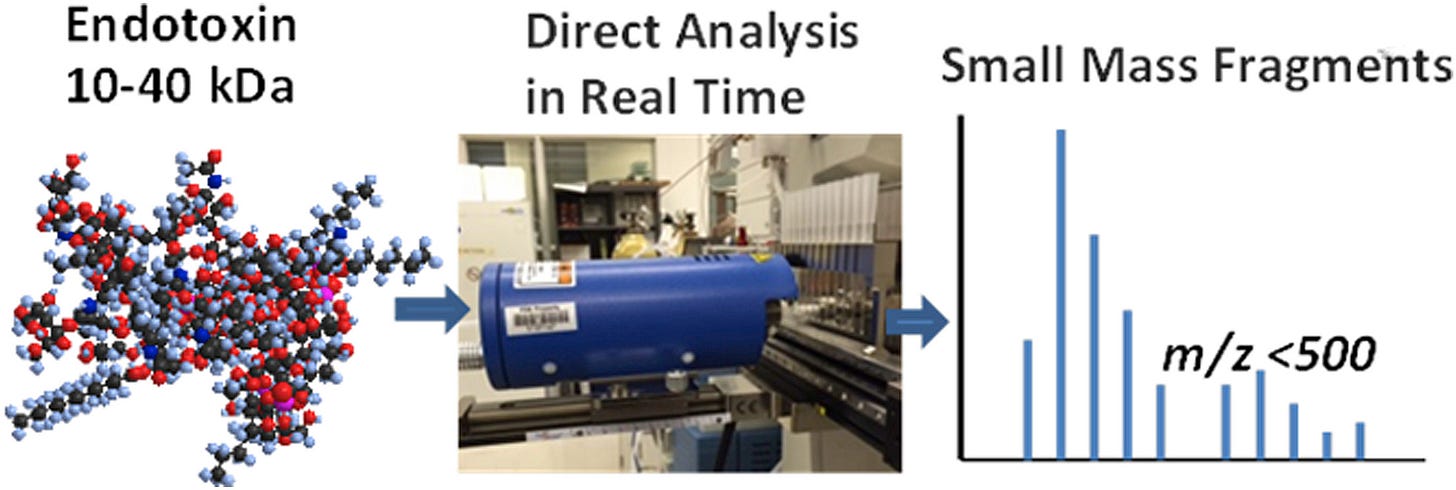
In 2016 a rapid test using Mass Spectrometry was reported by researchers at the FDA in collaboration with Nanjing Normal University that could detect 30 picograms/ml Endotoxin in aqueous solution.9
Notice the very large molecular mass Endotoxin they were interested in, ranging from 10 to 40,000 Daltons. The technique relies on recognition of a series of fragments derived by smashing the larger molecule and can detect both positively and negatively charged ions, measuring the mass to charge ratio (m/z).
MALDI-TOF
Advanced technology called Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry allows the very accurate measurement of large molecules and their fragments.
Dangers of Undetected Endotoxin
Low Endotoxin Recovery (LER) is the most commonly used term to describe the failure to detect the actual toxin level in a jab of interest and has led to FDA and other regulatory authorities holding workshops on the hazardous implications.1011
The hidden Endotoxin is also called “Masked”.12
Schwartz et al. demonstrated in 2017 that Citrate and detergents like Polysorbates can render the LAL test essentially useless as explained in their Figure 1.
Endotoxin Free E. Coli available but won’t be used
As discussed above, Pfizer wants the toxic Endotoxin, and especially its deadliest component in its jabs Lipid A to act as “Adjuvant” that they don’t have to pay for.
Genetic manipulation of E. coli to make a modified Lipid A, Lipid IVA that does not trigger an endotoxic response in humans was published in 2015.13
But other scientists are so concerned, they developed Entoxin free E. coli for the purpose of safer drug self-assembling nanoparticle protein manufacture.14
False Spikeopathy experiments
Failure to measure Endotoxin in commercial Spike Protein has led to many False conclusions in studies of Spikeopathy which in turn misdirects attempts to ameliorate Jab Damage.
SARS-CoV-2 Spike protein is Not pro-inflammatory in Human Primary Macrophages?
While there is no doubt the Synthetic SARS-CoV-2 Spike protein manufactured in jabbee cells, coded by synthetic mRNA in Covid19 jabs causes immense long-term damage to a generation, it can’t be responsible for the Adverse and sometimes Fatal reactions experienced in
Call to Action
We need politicians and lawyers to apply the necessary levers to get the hidden Endotoxin measurements and the analytical methods used from the regulatory authorities who have caused deliberate Septic Shock in the global mass jabbing tragedy. Revealed measurements will be shown to underestimate the poison.
My next Substack will cover another highly reliable Endotoxin test based on Monocyte Cytokine storm.
Needham BD and Trent MS. 2019. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. https://www.nature.com/articles/nrmicro3047
https://en.wikipedia.org/wiki/Mass_spectrometry
Sonesson A, et al. 1990. Comparison of the limulus amebocyte lysate test and gas chromatography-mass spectrometry for measuring lipopolysaccharides (endotoxins) in airborne dust from poultry-processing industries. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC184394/
Norbert Binding, Sabine Jaschinski, Sabine Werlich, Stefan Bletz and Ute Witting. 2004. Quantification of bacterial lipopolysaccharides (endotoxin) by GC–MS determination of 3-hydroxy fatty acids. https://pubs.rsc.org/en/content/articlelanding/2004/em/b309237b/
Casabuono AC, et al. 2012. Characterization of lipid A profiles from Shigella flexneri variant X lipopolysaccharide. https://www.infona.pl/resource/bwmeta1.element.wiley-rcm-v-26-i-17-rcm6306.
Pais de Barros J-P, et al. 2015. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. https://www.jlr.org/article/S0022-2275(20)35555-3/fulltext
Li H, et al. 2016. Rapid detection of bacterial endotoxins in ophthalmic viscosurgical device materials by direct analysis in real time mass spectrometry.https://www.sciencedirect.com/science/article/abs/pii/S0003267016311333
FDA 2012. Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers
https://www.pda.org/pda-europe/news-archive/full-story/2016/08/29/pda-s-ler-task-force-holds-its-first-workshop
Schwartz H, et al. 2017. Biological Activity of Masked Endotoxin. https://www.nature.com/articles/srep44750
Mamat U, et al. 2015. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-015-0241-5
Rueda F, et al. Structural and functional features of self-assembling protein nanoparticles produced in endotoxin-free Escherichia coli. https://link.springer.com/article/10.1186/s12934-016-0457-z