Busting open the LNPs to get to the Endotoxin
Low Endotoxin Recovery (LER) can be a boon to Big Pharma and the Regulatory Authorities they fund to "Pass" their grossly contaminated Jabs, but others are genuinely worried and try to overcome it.
A wide range of Jabs exhibit Low Endotoxin Recovery (LER) in attempts to measure the supertoxin using the Limulus Amebocyte Lysate (LAL) test developed by my distant cousin.1
Here I mention a few useful groups of researchers who highlight the problem and approaches they tried to understand if not beat LER.
A nice free recent paper by researchers in Germany working with Genentech, Inc., a member of the Roche Group2, looked at LER in an unnamed Monoclonal Antibody Drug including cryo-Transmission Electron Microscopy (cryo-TEM) to directly image Bacterial Endotoxin (from the rough-mutant strain Salmonella enterica Serovar Minnesota R60), and Small-Angle X-Ray Scattering (SAXS) to measure particle size distribution.
Here is one of their figures showing the difference betwen rough and smooth Endotoxin (LPS).
They used Mass Spectrometry to show the the Salmonella Endotoxin possesses hexa-acylated Lipid A as well as a tetra-acylated fraction (data not presented).
The caption reads:
Fig. 5. Model of LPS (Endotoxin) structures in water and in drug formulations impacted by Low Endotoxin Recovery (LER). A model of the LPS aggregate structure in aqueous suspensions in Endotoxin-free Water (EW) is shown on the left-hand side. LPS is assembled into extended tubular or ribbon-like aggregates with the cylindrical shaped acyl chain moiety of lipid A harbored inwards (yellow) and the sugar moiety directed outwards (indicated by blue ribbons).
These data are deduced from cryo-TEM measurements (Fig. 2F, Fig. S2E). In this conformation, the recognition structures for the LAL are fully expressed.
LPS aggregates in polysorbate/phosphate dispersions are shown on the right-hand side: single micelles forming an interconnected micellar network according to the cryo-TEM (Fig. 2F, Fig. S2E) and supported by DLS experiments (Fig. S2C, D). In this aggregation state, the recognition structures for LAL are hidden leading to the reduction or even absence of detection in LAL assay, the biological MAT assay and HEK cell assay. Biophysical characteristics of the LPS aggregates in EW, PL (The antimicrobial peptide Pep19-2.5 (Aspidasept®) with a sequence of GCKKYRRFRWKFKGKFWFWG) , and DP (unspecified monoclonal Drug Product) are summarized in the table below the respective models.
Here is part of another figure from Schromm et al. showing cryo-TEM images where you can see the Endotoxin.
The relevant part of the caption with my additions reads:
Cryo-TEM micrographs of the rough-mutant strain Salmonella enterica Serovar Minnesota R60 (LPS R60) in EW, PL, and DP. Lower micrographs show enlarged area of interest, indicated by the dotted squares.
Scale bars are 100 nm or 200 nm.
So you can see why Pfizer confessed it had no control of the amount of Endotoxin and its preferred “adjuvant” going through its crude filters with holes averaging 220 nm diameter.
Multifactorial Masking of Supertoxin
In 2018 Johannes Reich in Germany and coworkers in Japan demonstrated that it is necessary to dismiss simplistic approaches to quantifying the extent of masking the true supertoxin content of complex mixtures, as found in Covid19 and Childhood Jabs.3
They found that adjusting the ratios of complex-forming constituents, including surfactant, chelator (which grabs divalent Calcium or Magnesium catons, essential for the LAL test) and Endotoxin, and by testing the order in which the constituents are added, a new model for simulating masking kinetics could be produced.
Reich et al. used solutions containing Sodium Citrate and Polysorbate 20 to keep things simple.
For Endotoxin detection, a Limulus-based test (rFC) was used whereby the amount of fluorescence substrate (Amino-Methylcoumarin) released was measured fluorometrically at 440 nm (Excitation: 380 nm) with a FLx800 fluorescence microplate reader (BioTek Instruments GmbH, Bad Friedrichshall, Germany). The detection limit of the assay was 0.005 EU/ml. Compare this with the TGA detection limit of 5 EU/ml.
In some samples they spiked with 10 μL of Endotoxin from E. coli O55:B5 out of a 10,000 EU/mL stock solution. Before adding the Endotoxin spikes to the sample, the endotoxin stock solution was vortexed at 1400 rpm for 10 min using Multi Reax shaker.
They found that masking is independent of the initial Endotoxin concentration.
Fig. 2. Endotoxin recovery is independent of LPS concentration.
Endotoxin recovery is plotted as a function of incubation time.
Varying concentrations of Endotoxin were added to samples containing 0.05 (w/v) % Polysorbate 20 and 10mM Sodium Citrate. The black columns reflect 5000 EU/mL, grey columns 500 EU/ml and white columns 50 EU/ mL. The particular Endotoxin stock solutions for spiking were containing 50,000, 5000 and 500 EU/mL. For calculation of the data points the mean values of two individually prepared kinetics were used and the error bars reflects the corresponding standard deviations. For a better comparison of independent measurements, the data was normalized and the starting points were set to 100%. The mean of the initial measurements of 5000 EU/mL spike was 6993 EU/mL, 500 EU/mL spike was 640 EU/mL and 50 EU/mL spike was 72 EU/mL.
Time is a key variable. After 10 min of incubation, all recoveries were above 50% and after 45 minutes all recoveries were below 7%.
Tiny concentrations of Polysorbate 20 detergent collapsed the recovery of spike.
Endotoxin recovery over time in samples containing Polysorbate 20, sodium citrate and LPS is shown.
The different shaded columns reflect different Polysorbate 20 concentrations.
The set of black columns correspond to 0.0500 (w/v) % Polysorbate 20, the set of dark grey columns correspond to 0.0125 (w/v) % Polysorbate 20, the set of light grey columns correspond to 0.0008 (w/v) % Polysorbate 20 and the set of white columns correspond to 0% Polysorbate 20. The concentrations of spiked endotoxin (100 EU/mL) and sodium citrate (10 mM) were kept constant.
Reich et al also found that increasing the sample temperature can increase the masking of Endotoxin by increasing the reaction kinetics.
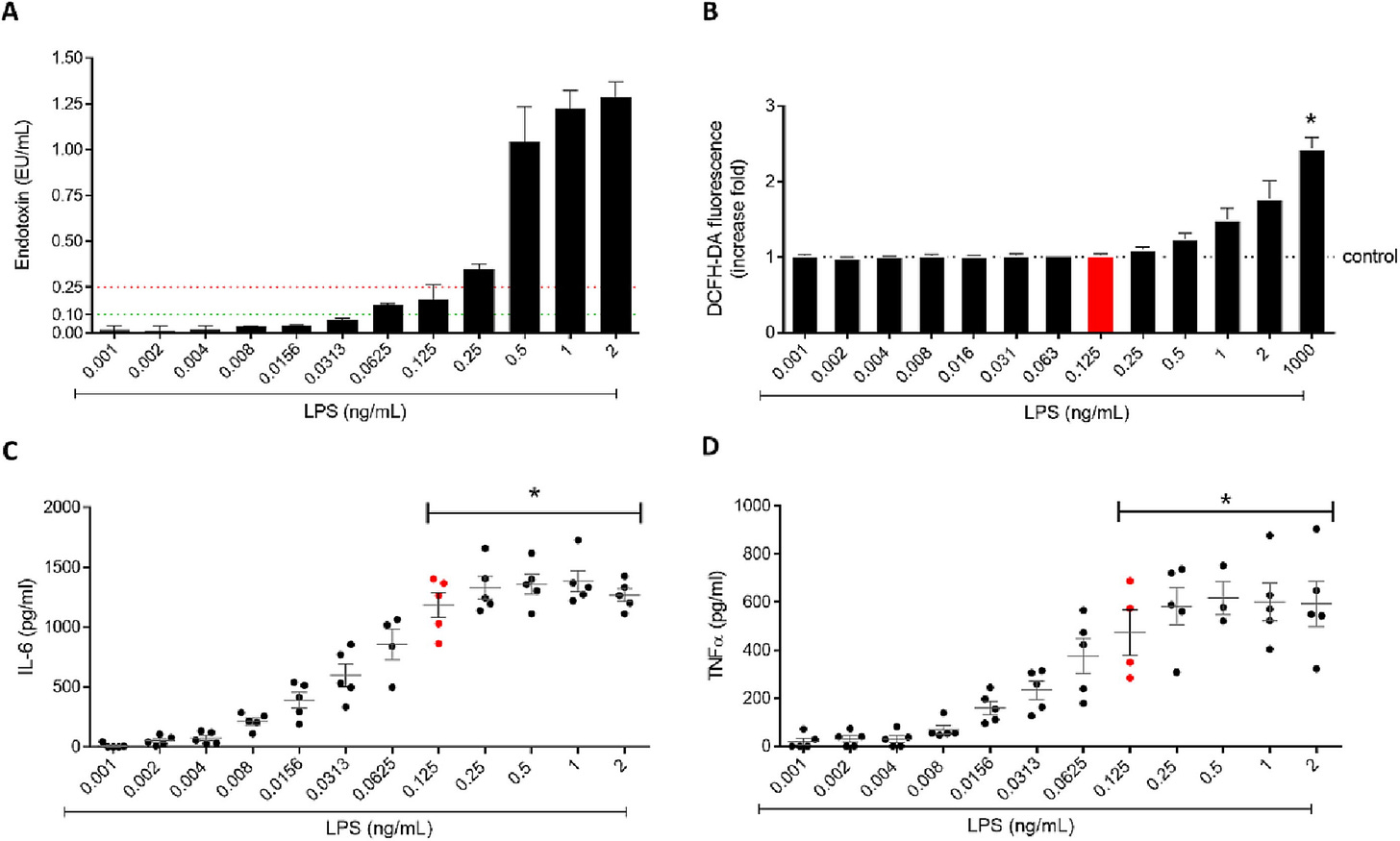
In 2023 a group in Portugal4 looked at problems using Chitosan Nanoparticles as Jab “Adjuvants”. Here is their graph showing non-linear response of Cytokines with increasing Endotoxin (LPS).
The caption reads:
Fig. 2. Increasing LPS concentrations effect on A) Endotoxin quantification by LAL assay, B) ROS production (DCFH-DA probe) by RAW 264.7 cells, C) IL-6 and D) TNF-α secretion by PBMCs. Results are expressed as mean ± SEM, n ≥ 3. * p < 0.05 compared to the negative control (Dunn’s multiple comparison test).
Here LPS = Lipopolysaccharide (from Salmonella enterica serotype minnesota); LAL = Limulus Amebocyte Lysate; ROS = Reactive Oxygen Species; DCFH-DA probe = 2’, 7’-DiChlorodiHydroFluorescein Diacetate; PBMCs = human Peripheral Blood Mononuclear Cells; IL-6 = Interleukin 6; TNF = Tumor Necrosis Factor; SEM = Standard Error of the Mean.
Decaethylene glycol monododecyl ether
Also known as C12E10 or Polyoxyethylene (10) lauryl ether, Decaethylene glycol monododecyl ether, is a nonionic surfactant sold by Merck. Its molecular formula is C32H66O11
It has interesting history and members of the Liposome Company moved to Merck after publishing their patent on its use to improve the Horsehoe Crab LAL measurement of Endotoxin trapped in Lipid Nanoparticles (LNPs).5
C12E10 can itself form micelles (LNPs) in water that can interact with and include alcohols.6
My friend Kevin McKernan is experimenting using C12E10 to measure Endotoxin in Covid19 jabs after his initial success using an alkaline detergent.7
Triton-X-100
Another non-ionic detergent has been successfully used by Didier Raoult to show that TGA and other authorities have been reporting False Low amounts of residual Plasmid DNA in Pfizer Jabs.8 He measured Pfizer Lot GJ7184 Residual Plasmid DNA 216 nanogram/dose, but when he added Triton-X-100 he observed an average of approximately 24 times greater - 5,160 nanogram/dose.
That means the Endotoxin content reported by TGA is masked too.
Unfortunately Triton X-100 interferes with the LAL Endotoxin test.
Further Reading
See previous posts mentioning LER.9101112131415
More references will be added.
Horseshoe Crabs Bleed for Pfizer Endotoxin Test
Pfizer is desperate to hide the amount of Endotoxin in their jabs caused by their use of E coli Bacteria in production. They rely on a test called LAL (Limulus Amoebocyte Lysate) extracted from the Blue Blood of Horseshoe Crabs, seen here being bled.
Andra B. Schromm, Wilmar Correa, Nicolas Gisch, Frank Steiniger, Walter Richter, Guillermo Martinez-de-Tejada, Klaus Brandenburg, Friedrich von Wintzingerode. 2024. Supramolecular assembly of micellar aggregates is the basis of low endotoxin recovery (LER) in a drug formulation that can be resolved by a whole blood assay. Biomedicine & Pharmacotherapy 173:116286 https://www.sciencedirect.com/science/article/pii/S0753332224001677
Johannes Reich, Hiroshi Tamura, Isao Nagaoka, Hubert Motschmann. 2018. Investigation of the kinetics and mechanism of low endotoxin recovery in a matrix for biopharmaceutical drug products. Biologicals 53:1-9.
João Panão Costa, Sandra Jesus, Mariana Colaço, Alana Duarte, Edna Soares, Olga Borges. 2023. Endotoxin contamination of nanoparticle formulations: A concern in vaccine adjuvant mechanistic studies. https://www.sciencedirect.com/science/article/pii/S0264410X23004875
Paul A. Harmon, Donna J. Cabral-Lilly, J. Craig Franklin, Robert A. Reed, Andrew S. Janoff. 1996. Detection of endotoxin levels in liposomes, lipid bilayers and lipid complexes. https://patents.google.com/patent/US6015716A/en
Sampsa Vierros and Maria Sammalkorpi. 2018. Effects of 1-hexanol on C12E10 micelles: a molecular simulations and light scattering study. https://pubs.rsc.org/en/Content/ArticleLanding/2018/CP/C7CP07511A
Measuring Endotoxin in Jabs with a Fluorescent Reaction
Here is a short article on the ThermoFisher Invitrogen Qubit Flex Fluorometer. It takes 8 plastic cuvettes (polymer a secret) held together as a strip for convenient handling and has a detection range of 0.01 – 1.0 EU/mL when using 50 μL of sample.Geoff Pain PhD is a reader-supported publication. To receive new posts and support my work, consider becomin…
Didier Raoult. 2024. Confirmation of the presence of vaccine DNA in the Pfizer anti-COVID-19 vaccine. https://hal.science/hal-04778576v1
FDA detected Endotoxin by Mass Spectrometry
As mentioned in earlier posts Gram Negative Bacterial Cell fragments known as Endotoxin and its supertoxin Lipid A are deliberately incorporated into the Pfizer Covid19 and Novavax jabs and are also present in AstraZeneca, Janssen, Moderna jabs because no technology was applied to remove them.
AstraZeneca Endotoxin Test Meaningless
In a previous articles I have shown that AstraZeneca was withdrawn because it was killing a higher proportion of people that Pfizer or Moderna and causing Eye Damage.
Lipid Nanoparticles, Endotoxin and Detergent Micelles
We know that Covid19 Jab manufacturers are desperate to stop Jabbees learning how much Endotoxin is in the various Batches and Brands of the toxic soups.
Endotoxin content of Pfizer Multiple Myeloma Jab Elrexfio
We know that Endotoxin and its Lipid A cause Myeloma.
Endotoxin content of Jabs underreporting caused by Aluminium
Regulatory authorities never release their Vaccine Endotoxin testing results for fear that independent scientists could evaluate deficiencies and inconsistencies in their methods.
Vaxelis Endotoxin content a Dark TGA Secret
Looking for any information at all on the Endotoxin of Vaxelis jabs approved by the TGA in March 2022. I know it is in there.
Jemperli Dostarlimab Deaths most likely due to Massive Endotoxin Dose
I was alerted by a friend on X (Formerly Twitter) that Jemperli, a brand name for GSK Dostarlimab, was being hailed as a “Cure for Cancer” with “100% Remission”.