Endotoxin in Pfizer ABRYSVO Respiratory Syncytial Virus Jab a Deadly Secret
Watch this space because 5 of the 12 CBER reviewers refused to allege the safety and effectiveness of ABRYSVO and Deaths are being reported
Just a quick note to show that Endotoxin is prime suspect in the ABRYSVO RSV jab that is already claiming casualties as reported by Jessica Rose1 and others.
Here is page 9 of the CBER review that must surely inspire confidence and informed consent in Jabbees, if only they could see what is hidden.2
ABRYSVO RSV jab consists of 120 mcg of lyophilized, recombinant antigen derived from theRSV fusion (F) surface glycoproteins of the two RSV subgroups, RSV-A (60 mcg) and RSV-B (60 mcg) stabilized in the pre-fusion trimeric conformation (RSVpreF) and is administered intramuscularly (IM) as a single dose (0.5 mL).
Each dose of ABRYSVO also contains 0.11 mg Tromethamine3, 1.04 mg Tromethamine Hydrochloride, 11.3 mg Sucrose, 22.5 mg Mannitol, 0.08 mg Polysorbate 804, and 1.1 mg Sodium Chloride (Salt) per 0.5 mL.
The jab is manufactured in genetically engineered Chinese Hamster Ovary cell lines (CHO) grown in suspension culture, which will contribute most of the Endotoxin, known to produce Premature births, Fetal and Maternal Death.5
On Twitter Sandeep Chakraborty6 showed the grim reaper is active:
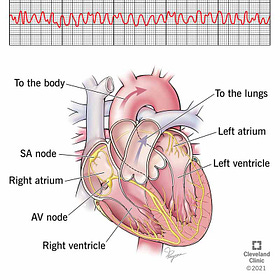
Predictable Cardiac Damage to RSV Jabbees
The CBER report states:
Within 1 month after vaccination, there was a numerical imbalance observed in events under the SMQ Cardiac arrythmia, with 21 events reported by 17 participants (0.1%) in the RSVpreF group and 8 events reported by 7 participants (<0.1%) in the placebo group. This imbalance was primarily driven by events of atrial fibrillation (10 events in 10 participants [<0.1%] in RSVpreF group compared to 4 events in 4 participants [<0.1%] in placebo group), of which 4 in the RSVpreF group and 3 in the placebo group were serious adverse events.
CBER says:
The committee members emphasized the need for postmarketing surveillance to continue to assess Guillain-Barré Syndrome (GBS), potential immune-mediated diseases (pIMDs) in general, and atrial fibrillation.
Read why Atrial Fibrillation is expected.7
February 2025 Update
On 7 January 2025 US FDA issued a warning8 Guillain-Barré Syndrome9 risk from this poison ABRYSVO, as relayed by JAMA.10 It was only a matter of time.
The warning also applies to GSK Arexvy that I covered earlier undeer my Cervarix article.11
Sen Goutam et al. 31 May 2023. Summary Basis for Regulatory Action. Proprietary Name: ABRYSVO, Proper Name: Respiratory Syncytial Virus Vaccine
Tromethamine is a Hazardous Substance in Jabs that Must be Banned
Tromethamine is an Endocrine Disruptor and causes Anaphylaxis. In Australia the Therapeutic Goods Administration, acting on the advice of The Advisory Committee on Vaccines, and The Australian Technical Advisory Group on Immunisation, approved a modified Pfizer injection for young Children aged 5-11 years old, using Tromethamine, also known as Tris, Trom…
Polysorbate 80 in Covid19 Jabs a Major Hazard
I was pleased to find a major thread on Twitter from 2021, where I discussed the hazards of Polysorbate 80 in Covid19 jabs, remains intact after Elon Musk restored my account. Polysorbate is used in AstraZeneca and Janssen jabs. So I can build on that with updates, meanwhile sharing early.
Abortion, Preeclampsia and Placenta Damage by Pfizer jabs are expected from the vial Endotoxin
Analysis of VAERS Data My good friend OpenVaet has done a deep dive into the acknowledged under-reported US VAERS database to 22 August 2022 and has produced an an excellent interactive guide to the impact of the Janssen, Moderna and Pfizer Covid19 jabs on Pregnancies.
Atrial Fibrillation is the most common type of Tachycardia, Anaphylaxis leading to Sudden Death caused by Endotoxin in mRNA Jabs
In recent articles I covered Heart Damage and Deaths arising from Positive Feedback Loop generation of damaging microRNAs, and Anaphylaxis Deaths, all caused by Endotoxin that arises from the use of E coli bacteria in production. Tachycardia with associated Fever, Hypotension and Lymphopenia is the expected outcome from injection of Endotoxin, as has bee…
FDA Requires Guillain-Barré Syndrome (GBS) Warning in the Prescribing Information for RSV Vaccines Abrysvo and Arexvy. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-guillain-barre-syndrome-gbs-warning-prescribing-information-rsv-vaccines-abrysvo-and
Guillain-Barré Syndrome expected from Pfizer Endotoxins
Facial and peripheral paralysis were recognized early as Neurological Adverse Reactions after Pfizer jabbing trials. These were included among Neurological Adverse Events of Special Interest (AESIs including Demyelination, i.e. destruction of the protective sheath on our Nerves).
Samantha Anderer. 21 February 2025. FDA Issues Warning of Guillain-Barré Syndrome Risk for 2 RSV Vaccines. https://jamanetwork.com/journals/jama/fullarticle/2830753
Cervarix HPV jab Deaths and Injuries caused by Endotoxin Lipid A
Children have been killed and maimed by Cervarix jab which is composed of recombinant C-terminally truncated HPV-16 L1 and HPV-18 L1 proteins (20 micrograms each) in virus-like particles (VLPs) adjuvanted with AS04 adjuvant system, composed of an Aluminium Hydroxide Al(OH)3 (500 micrograms) and 3-O-desacyl-4'-MonoPhosphoryl Lipid A (MPL, 50 micrograms).