Guillain-Barré Syndrome expected from Pfizer Endotoxins
Pfizer jabs contain Endotoxins from gram-negative Bacteria used in production which cause paralysis via Molecular Mimicry between Bacterial Glycoconjugates and Peripheral Nerve Gangliosides.
Facial and peripheral paralysis were recognized early as Neurological Adverse Reactions after Pfizer jabbing trials. These were included among Neurological Adverse Events of Special Interest (AESIs including Demyelination, i.e. destruction of the protective sheath on our Nerves).
There was 1 confirmed report of GBS in the Pfizer trials.1
There were 24 reports of GBS in the first 90 days after Pfizer mass jabbing began.2
Pfizer reported 226 cases of GBS in its June 2021 report.3
To 15 April 2022, Pfizer reported 1,461 cases of Guillain-Barré Syndrome (GBS).4 This amounted to 0.11% of all 1,348,079 case reports known to the company by that date.
By June 2022 Pfizer reported 1,559 cumulative cases of GBS5, an increase of 500 cases per month!
Endotoxins from gram-negative E coli Bacteria, as used in Pfizer jab production, cause paralysis via molecular mimicry between bacterial glycoconjugates and peripheral nerve gangliosides.6
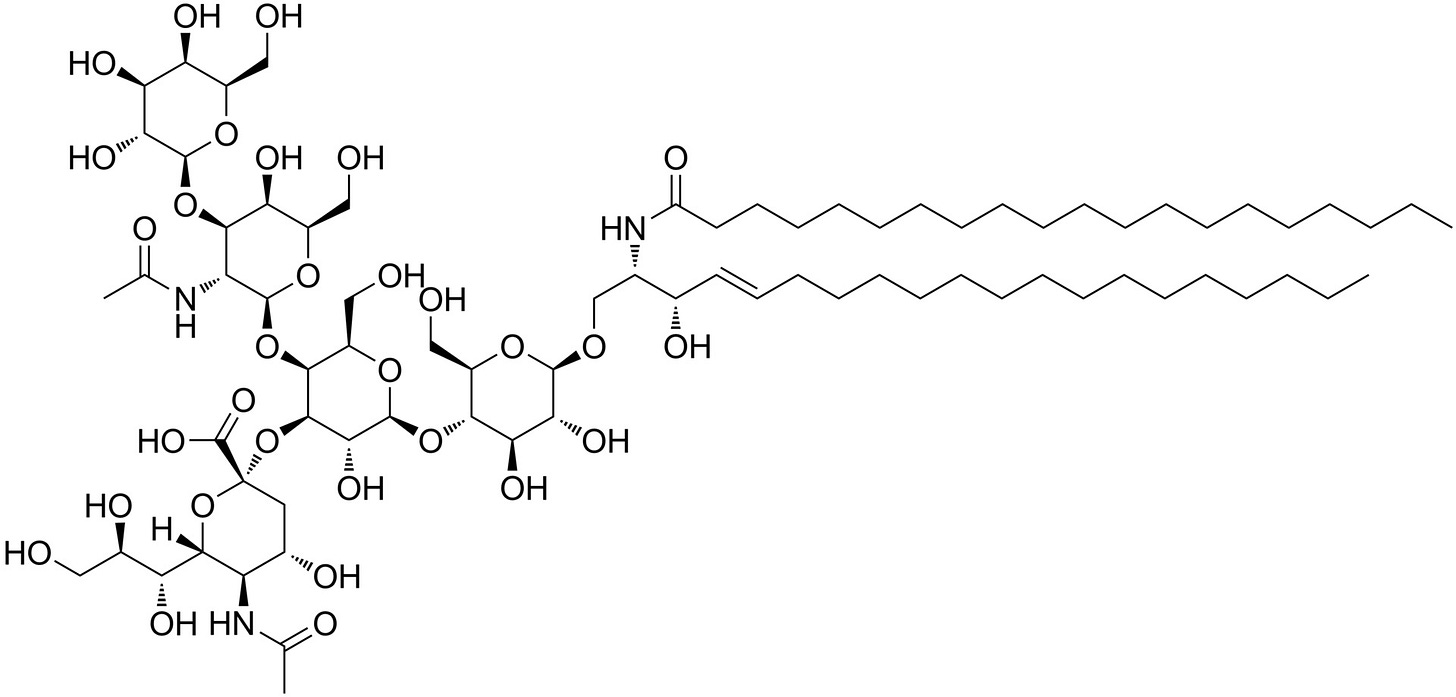
Note the similarity in structure of GM1 Ganglioside7 shown below to the Lipopolysaccharide (LPS, Endotoxin), known as Lipid A.8
GBS is just one of numerous Neurological diseases caused by the Pfizer jabs, many ultimately causing Death. Pfizer excluded people with family history of Guillain-Barré Syndrome from some of its later trials.
A case report describes in detail a 16-year-old boy who suffered GBS after his 3rd jab.9
Influenza vaccination and Guillain Barré syndrome
Influenza jab Endotoxin levels have been correlated with GBS cases reported to VAERS.10
Median onset of GBS following influenza vaccine was 12 days (interquartile range, 7 days to 21 days). There was an increased risk of acute GBS (relative risk, 4.3; 95% confidence interval CI, 3.0 to 6.4) and severe GBS (relative risk, 8.5; 95% CI, 3.7 to 18.9) in comparison to an adult Tetanus–Diphtheria (TD) vaccine control group.
Influenza vaccines contained from a 125- to a 1250-fold increase in Endotoxin concentrations in comparison to the TD vaccine control and Endotoxin concentrations varied up to 10-fold among different Lots and manufacturers of Influenza vaccine.
Aged suffer more from Endotoxin Neuroinflammation
In 2005, it was shown that E coli Endotoxin from strain 0127:B8 cause inflammation in male BALB/c mice. There were 38 genes found in the Brains associated with inflammation and aged mice suffered more damage.11
Urinary tract infection with E Coli has been identified as a trigger for recurrent GBS in a 75-year-old woman with a prior history of Acute Motor Axonal Neuropathy (AMAN, a subtype of GBS).12 She suffered weakness of limbs and areflexia following 10 days of fever, frequency, and dysuria. This lady partially recovered after treatment with intravenous immunoglobulin with ceftriaxone.
It would be interesting to look at the age distribution of GBS Pfizer jab victims.
Pfizer reports GBS in RSV Jab trial
The madmen and madwomen at the US Food and Drug Administration (FDA) are fully aware of 1 case of GBS with onset 7 days after vaccination and a case of Miller Fisher syndrome (considered a variant of GBS) with onset 8 days after vaccination, were caused by Pfizer’s Respiratory Syncytial Virus (RSV) vaccine during its clinical trial.13
See also an article by Darrell Ricke on GBS with many useful references, discovered after I wrote this piece.14
And thanks to Michael Nevradakis15 for telling us about a new epidemiology level report from South Korea, US, Australia, France, Iran and the UK.16
Joseph Gehrett, MD; Barbara Gehrett, MD; Chris Flowers, MD; and Loree Britt. 2023. https://dailyclout.io/report-57-542-neurological-adverse-events-95-serious-in-first-90-days-of-pfizer-mrna-vaccine-rollout-16-deaths-females-suffered-aes-more-than-twice-as-often-as-males/
Pfizer 2021. COVID-19 mRNA vaccine (nucleoside modified) EU Periodic Safety Update Report (PSUR) 1, covering the 6 month period from 19 December 2020 through 18 June 2021
APPENDIX 2.1 Cumulative Number of Case Reports (Serious and Non-Serious, Medically Confirmed and Non Medically-Confirmed) from Post-Marketing Data Sources, Overall, by Sex, Country, Age Groups and in Special Populations and Summary Tabulation by Preferred Term and MedDRA System Organ Class BNT162B2 - ALL Reporting Period: Through 15-APR-2022.
APPENDIX 2.2: Cumulative and Interval Summary Tabulation of Serious and Non-Serious Adverse Reactions from Post-Marketing Data Sources BNT162B2 Cumulative Reporting Period: Through 18-JUN-2022 Interval Reporting Period: 19-DEC-2021 Through 18-JUN-2022
Ang, CW. et al. 2002. https://journals.asm.org/doi/10.1128/IAI.70.3.1202-1208.2002
https://en.wikipedia.org/wiki/Ganglioside
Finsterer J. Guillain–Barre Syndrome After the Third BNT162b2 Dose in an Adolescent Without Side Effects After the First and Second Jab. https://journals.lww.com/jcnmd/Citation/2023/03000/Guillain_Barre_Syndrome_After_the_Third_BNT162b2.6.aspx
Geier MR, et al. 2003. Influenza vaccination and Guillain Barré syndrome. Clinical Immunology 107:116–121.
Godbout JP, et al. 2005. https://faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fj.05-3776fje
Jo Y-S, et al. 2017. https://jkms.org/DOIx.php?id=10.3346/jkms.2018.33.e29
FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) 24 February 2023. Briefing documents. Pfizer 2023. Respiratory Syncytial Virus Vaccine (Proposed Trade Name: Abrysvo).
Yi Deun Jeong, Seoyoung Park, Sooji Lee, Woojin Jang, Jaeyu Park, Kyeongmin Lee, Jinseok Lee, Jiseung Kang, Raphael Udeh, Masoud Rahmati, Seung Geun Yeo, Lee Smith, Hayeon Lee and Dong Keon Yon. 2024. Global burden of vaccineassociated Guillain-Barré syndrome over 170 countries from 1967 to 2023. https://www.nature.com/articles/s41598-024-74729-2