Covishield version of AstraZeneca Jab
Starting to delve into the strange happenings related to collaboration between AstraZeneca and The Serum Institute of India
Covishield is a hot topic for hundreds of millions of Jabbees.
Starting my delve into its Harms.
There is the question about where the Covishield was manufactured when Emergency Use Authorization was sought in Europe.1
Covishield was not accepted for the EU travel pass when over 300,000,000 people had been jabbed.2
There are reports that jab certificates carried a propaganda message from the PM.3
There was a WHO alert put out that Fake versions of Covishield were circulating in Uganda, India and Myanmar.4
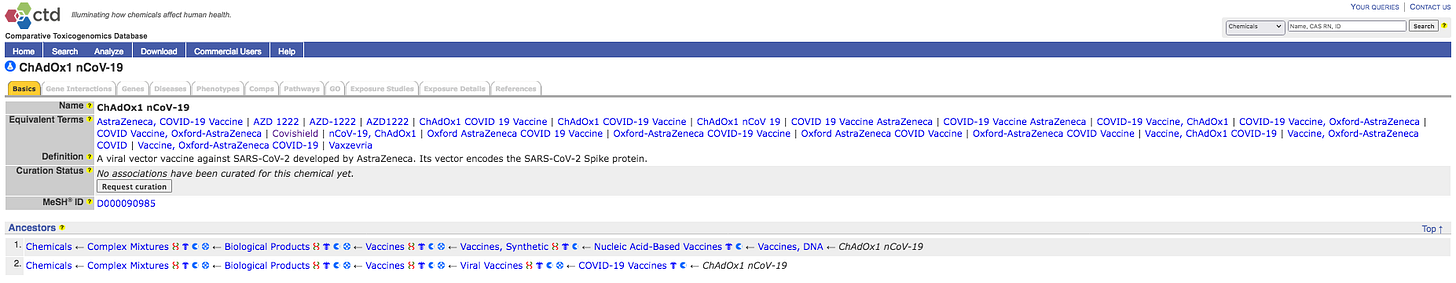
Unfortunately the US CTD has Zero entries for Covishield or AstraZeneca Harms, but note that one might request curation. Perhaps a lawyer could do that?
We know hundreds of peer-reviewed papers on the AstraZeneca jab have been published.
I have a large AstraZeneca collection thanks to the New South Wales Health Department Critical Intelligence Unit weekly broadcasts, but I want to focus on the version with the Covishield Label. Click on image to expand.
A number of other authors have written about Covishield here on Substack, so I will add them as references later if relevant to Harms.
Harms caused by Covishield
A small study from India by jab enthusiasts looked at Covid19 patients who were jabbed versus unjabbed in the Princess Krishnajammanni Trauma Care Centre (PKTCC), Mysore, which was converted to a designated COVID hospital between April 2021 to July 2021.5
Jabbed patients suffered more Vomiting, with 3.9% and 4.3% of single or fully vaccinated patients reported vomiting compared to 1.3% of patients who had not received any vaccines. The authors did not understand why, but I can point to the likely cause.6
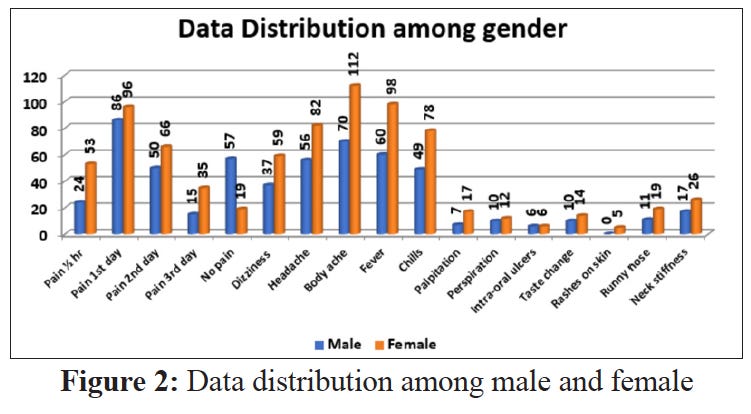
A 2021 paper concentrated on Adverse Events reported from the 1st dose of Covishield given to 358 government officials. It was conducted by online survey from 3rd to 10th February 2021 with a list of solicited symptoms.7
Pain
Dizziness
Headache
Body Ache
Fever
Chills
Palpitations
Perspiration
Intra-oral Ulcers
Taste Change
Rashes on Skin
Runny Nose
Neck Stiffness
Females were found to experience adverse events more than males, and younger people suffered more.
Deaths from Covishield
Recently there has been renewed Legal Action in India by relatives of people killed by Covishield and a new website established for jab victims and their families to report adverse events.8
The recent admission in UK and elsewhere that AstraZeneca directly caused Deaths should assist Covishield claimants.
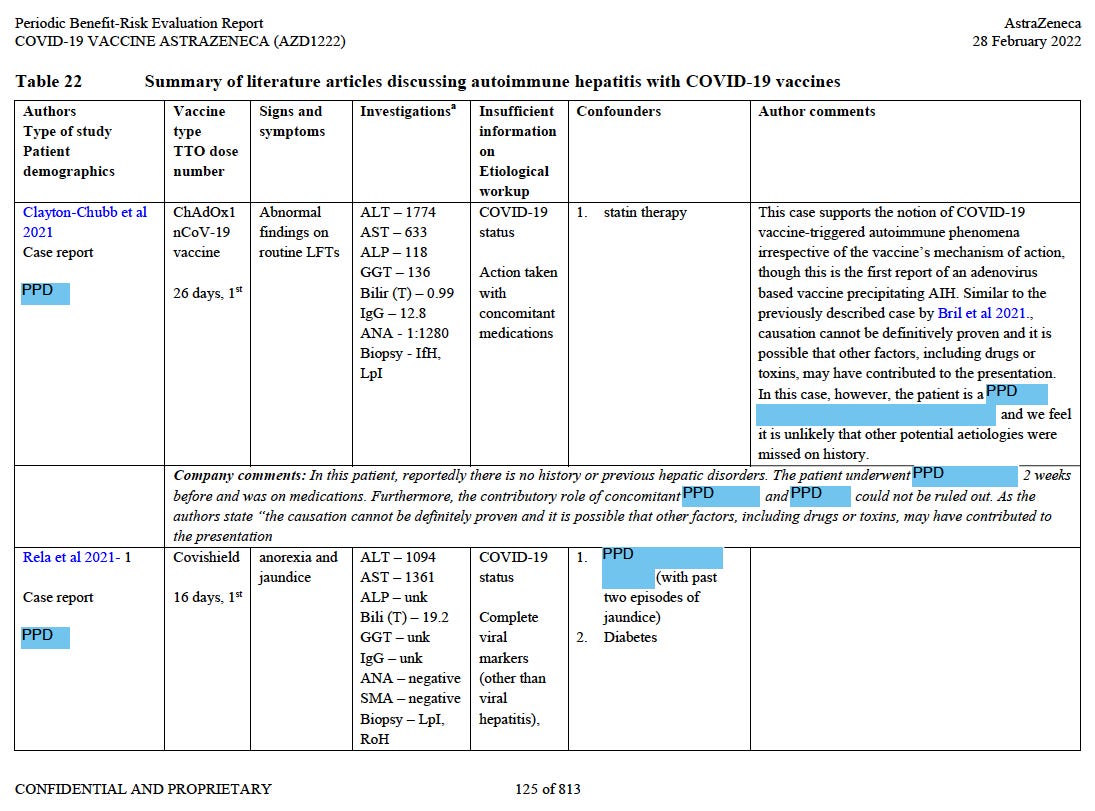
We can find a few Covishield jab victims mentioned in the mandatory Periodic Safety Update Reports from AstraZeneca.910
See the expected11 Rapid onset Autoimmune Hepatitis with some clinical data.
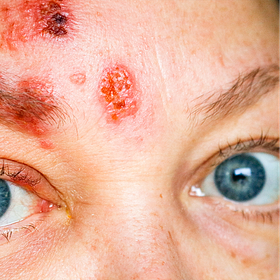
Eye Damage was common within days of Covishield. This is one case of many discussed in the PSURs based on peer-reviewed case studies.
Singh et al 202212 ( case ID Redacted) In this case report article authors present a case of herpes simplex (HSV) retinitis in 29-year-old male two days following the first dose of the Covishield vaccine. Since the blood serologies were all negative, a diagnostic vitrectomy was performed and the vitreous sample for viral PCR came positive for HSV-1 . At 6-week follow-up, the patient improved with a near-complete resolution ofretinitis after the course of antiviral therapy.
AstraZeneca comment: There is a limited information on patient concomitant medications, medical history, and presence of any alternate viral reactivation triggers that precluded a proper assessment. The case was assessed as Possible (with limited information) according to WHO-UMC criteria.
We know Endotoxin triggers Dormant Virus reactivation.13 Blindness often follows in one or both eyes.14
I look forward to reading your Covishield contributions and expanding later.
https://www.bbc.com/news/world-asia-india-57633980
https://qz.com/2026656/the-eu-digital-covid-certificate-excludes-the-covishield-vaccine
https://thewire.in/government/india-only-nation-to-use-covid-vaccination-certificate-to-push-cult-of-leader-ruling-party
https://www.lowyinstitute.org/the-interpreter/blockchain-solution-covid-19-vaccine-scams
Ganesh Korishettar, Prashanth Chikkahonnaiah, SubbaRao V. Tulimilli, Siva Dallavalasa, Shashidhar H. Byrappa, SubbaRao V. Madhunapantula and Ravindra P. Veeranna. 2022. Assessment of Clinical Profile and Treatment Outcome in Vaccinated and Unvaccinated SARS-CoV-2 Infected Patients.
Diarrhoea and Vomiting brought to you by Pfizer
In one of the largest per capita population studies of Harms caused by Pfizer jabs, the AusVaxSafety telephone or email survey of over 5 million Jabbees (no more than 3 days after getting the poison), 1 in 9 people reported Gastrointestinal symptoms, including Diarrhoea, Vomiting, Nausea and Abdominal Pain after the second jab. This was considerably mor…
Srishti Jain, Sandhya Jain and S.V Sai Prasad. 2021. Coronavirus Vaccine: A Cross-Sectional Study of Its Adverse Events. https://www.researchgate.net/publication/355664459_Coronavirus_Vaccine_A_Cross-Sectional_Study_of_Its_Adverse_Events
Swati Bharadwaj. Kin claim deaths due to Covishield, to sue Serum Institute of India. https://timesofindia.indiatimes.com/city/hyderabad/kin-claim-deaths-due-to-covishield-to-sue-serum-institute-of-india/articleshow/110046824.cms
AstraZeneca. 28 February 2022. COVID-19 Vaccine (AZD1222) Periodic Benefit-Risk Evaluation Report.
AstraZeneca. 25 August 2022. COVID-19 Vaccine (ChAdOxl-S [recombinant]) Periodic Benefit-Risk Evaluation Report.
Rapid Liver Failure after Pfizer jabs most likely due to miR-155 upregulation by Endotoxin
In a previous article I showed how Heart Damage caused by mRNA jabs can be explained by the catalytic effects of upregulation of the microRNA molecule known as miR-155. Liver Failure during the Trial Pfizer knew from its clinical trial and first 90 days of mass jabbing that severe and Fatal Liver Failure was a clear signal of systemic assault by its produ…
Singh, J., A. More, S. B. Shetty, P. Chaskar,A. Sen. Herpes simplex virus retinitis following ChAdOxl nCoV- 19 (Covishield) vaccination for SARS CoV 2: A case report Ocular Immunology and Inflammation 2022.
Shingles caused by Pfizer Jabs - Endotoxin or Spike?
In August 2021, Queensland Health decided to tell us all about Shingles with pictures. They used the occasion to promote Zostavax® vaccine which “is available on prescription to people aged 50 to 69 years and from 80 years, but it must be paid for by the patient.”
Blindness from AstraZeneca Jabs
I was given, by an Australian doctor, comparative data from the UK Government Yellow Cards Adverse Events Database, showing that there were more symptoms of Eye Disorder for AstraZeneca jabs compared to Pfizer or Moderna Covid19 jabs as a proportion of total reports.