Meningococcal Jab Adverse Events linked directly to Endotoxin in 1978
Hot lots were observed in MenA Jab with Endotoxin content varying by a factor of 20 in Finland.
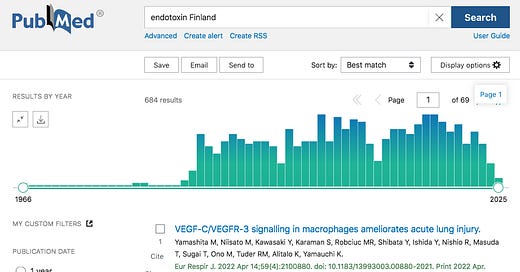
I was mentioning to a friend last week that Finland has produced a lot of Endotoxin research.1
Searching PubMed for Endotoxin Finland, I found 684 peer reviewed papers, 42 of which also mention vaccine.2
For Lipopolysaccharide Finland, I found 872 papers, of which 53 also mention vaccine.
For LPS Finland, the search yields 553 papers, of which 20 also mention vaccine.
Rather than bore readers with a mind blowing overload of science, I thought it best to just show the definitive research in one paper from 47 years ago finding Hot Lots in a Meningococcal Jab.3
The research was funded by National Institutes of Health, USA (Contract 1AI 52502) and examined 5 different Meningococcal Jab Lots made by Merck Sharp & Dohme Research Laboratories, and Institut Merieux France.
Here is their data in 2 Tables. The dose was 30 μg of capsular polysaccharide (a polymer of N-ace@ mannosamine phosphate) purified from Neisseria meningitidis strain A5, except for infants from three to five months of age who received only 20 μg.
Table I compares Lot Numbers with Adverse Reaction rates. But the figures are fiddled to make the disaster not look too bad with evidence of Vaccine Enhanced Disease :
One third of the children with high fever also had symptoms of respiratory infection.
Since no child with fever was vaccinated, it is possible that the fever with respiratory infection was related to vaccination.
However, in the following analysis only fever without signs of infection is counted.
Table II compares Adverse Reaction rates for Meningococcal Jabs with other types of Jab.
Comparative Endotoxin Testing
They were only able to perform relative contamination level measurements.
All the vaccines reacted strongly both in the Limulus and the rabbit pyrogenicity tests. Since the Limulus test was calibrated with E. coli and not with meningococcal endotoxin, and since the two endotoxins may differ in their ability to coagulate the amebocyte lysate, the figures probably do not give the exact concentrations of meningococcal endotoxin.
However, they seem to give fair estimates of the relative endotoxin content of the vaccine lots. Thus lot 590 had the lowest, and lot 572 the highest Endotoxin content; the former corresponds to the activity of 0.6 μg and the latter to that of 14 μg of E. coli Endotoxin per 100 μg of polysaccharide.
The rabbit pyrogenicity test gave results consistent with the Limulus assay.
So they estimated 120 to 400 nanogram amounts of Endotoxin caused the Adverse Reactions in the Finland meningococcal jabbing. That far exceeded the levels used in Human volunteer experiments that I have described in other articles that cause immediate rise in Brain and Body temperature.
Symptoms due to Endotoxin
Peltola and coworkers reported:
Erythema less than one inch in diameter (30%)
Erythema larger than one inch (12%)
Tenderness of the upper arm (25%)
Tender nodes in the axilla (2%) i.e. Lymphadenopathy
Elevated temperature, Fever
High fever (over 38.5 degrees C), was reported in 294 (1.8%) of all children
Chills
Trembling
Pallor
Irritability
Reduced activity
There were three possible allergic reactions. A one-year-old girl developed a morbilli-form rash after primary vaccination and within 30 minutes of a booster dose she became restless and flushed after which she vomited.
Her pulse rate was 140/minute and systolic blood pressure 80 mm Hg, but she remained conscious. After an injection of epinephrine and hydrocortison she recovered promptly.
A boy of five years started to complain of sore throat and abdominal pain two hours after MenA Vaccination and became sweaty and pale. He received hydrocortisone and recovered promptly.
A girl of four years was hospitalized because of an urticarial reaction, which had appeared two days after MenA vaccination. No other cause for the reaction was apparent; the child was known to be atopic.
Remember that Meningitis is caused by Endotoxin in Pfizer Covid19 Jabs and the mechanism is known.4
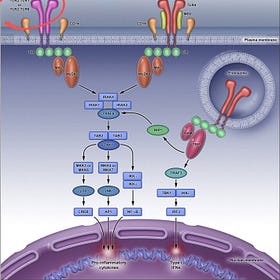
It is a TLR25 TLR56 and TLR9 disease.7
The supertoxic part of Endotoxin, and Pfizer’s preferred “adjuvant” Lipid A causes Meningitis.8
Meningitis was reported from Endotoxin contaminated surgical material.9
I hope you found that old 1978 paper from Finland interesting.
https://x.com/FluoridePoison/status/1885467536285983066
https://pubmed.ncbi.nlm.nih.gov/?term=endotoxin+Finland
Heikki Peltola, Helena Käyhty, Tapani Kuronen, Nora Haque, Seppo Sarna, Ph.D., and P. Helena Mäikelä. May 1978. Meningococcus group A vaccine months to five years of age. Adverse reactions and immunogenicity related to endotoxin content and molecular weight of the polysaccharide. The Journal of PEDIATRICS. 92(5):818-822. https://www.jpeds.com/article/S0022-3476(78)80165-6/abstract
Meningitis caused by Endotoxin Lipid A in Covid19 Jabs
As I reported earlier, Supertoxin Endotoxin Lipid A in Covid19 jabs travels in a matter of seconds to minutes, immediately entering the Brain with devastating consequences.
TLR2 Diseases caused by Bacterial Lipoprotein in Jabs
Recently I reported on Foster Coulson company Qu Biologics that has patented Jabs containing extracts of killed Bacteria. I covered some of the harms caused by hitting your TLR5 and now I focus on TLR2 induced harms which originate from part of the Bacterial Cell Wall known as Lipoprotein or Lipopeptide.
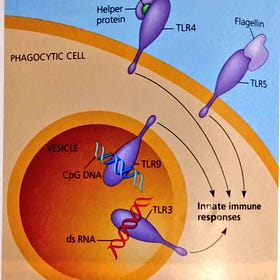
TLR5 Diseases from deliberate use of Bacterial Flagellin in Jabs
In previous posts I have mentioned the Human Toll-like Receptor 5 (TLR5) that reacts to the “dangly bits” emanating from Bacteria, in contrast to the Endotoxin (Lipolysaccharide) fragments of the cell wall. In this figure we see that TLR5 and TLR4 are on the surface of your cells, whereas DNA contamination of the jabs triggers reactions via TLR9 and dou…
TLR9 Diseases from Endotoxin and DNA in Jabs
Previously I have covered the impact of Endotoxin, either as a contaminant, or deliberately added to injections on a range of Toll-Like Receptors (TLRs).
Lipid A in Jabs, the Supertoxin they won't report
In numerous Substack articles I have mentioned various aspects of the Lipid A Endotoxin fragment. Now that there is greater public awareness, Parliamentary investigations and Court cases involving the Septic Shock induced in billions of people by this agent, this article will bring together some of the highlights.
Endotoxin forced Recall of Durepair Dura Regeneration Matrix
Australia’s Recall notice demanded return of all unused product in the possession of Medtronic customers. It was used for repair of the dura mater during neurosurgical procedures.







“Probably”; “seem to give”; “fair estimate”; “may differ”.
This is science?
What weasel-language. What cavalier disregard for their victims’ health. What very dangerous people.
Thank you, Geoff.