Endotoxin forced Recall of Durepair Dura Regeneration Matrix
In 2023 there was a worldwide recall of a surgical collagen material derived from Fetal Bovine skin cells due to the release of product with out-of-specification Endotoxin levels.
Australia’s Recall notice demanded return of all unused product in the possession of Medtronic customers. It was used for repair of the dura mater during neurosurgical procedures.
Photo credit.1
All Durepair products manufactured at a contract manufacturing facility had identified issues with in-process and finished goods Endotoxin testing.2
The company had received 18 complaints.
Integra LifeSciences Corporation Manufacturer
The Fetal Bovine Collagen used in the product was manufactured by Integra LifeSciences Corporation at 7 Elkins Street, Boston, where the FDA conducted an inspection of medical device operations from March 1 through May 17, 2023.
This resulted in a Warning Letter, with (b)(4) redactions3 which says in part:
This inspection revealed that the above devices are adulterated within the meaning of section 501(h) of the Act, 21 U.S.C. § 351(h), in that the methods used in, or the facilities or controls used for, their manufacture, packing, storage, or installation are not in conformity with the current good manufacturing practice requirements of the Quality System regulation found at Title 21, Code of Federal Regulations (CFR), Part 820.
We received your response dated June 8, 2023 from Susan Krause, Corporate Vice President, Chief Quality Officer, Integra Lifesciences Corporation, which responded to the Form FDA 483, List of Inspectional Observations issued to your firm on May 17, 2023. On May 23, 2023, our office also confirmed that TEI initiated a recall of all finished goods manufactured within expiry from March 1, 2018 through May 22, 2023, (RES 92481). We also understand that you have committed to not distributing product until the TEI Bioscience site is operating in substantial conformity with Quality System regulations (QSR). We address your June 8, 2023 response below.
These violations include, but are not limited to, the following:
1. Failure to establish and maintain procedures to control product that does not conform to specified requirements, as required by 21 CFR 820.90(a). Specifically:
• On June 19, 2019, your firm released a lot of Durepair,# 1904005 for distribution that indicated a bacterial endotoxin (BET) result of 9.98 EU/Device. This result exceeded your acceptance criteria of (b)(4) device as specified in your procedure, QCP-037 - Performance of the (b)(4) Endotoxin Assay.
The device history record for this lot of product showed that the lot was approved and released by your firm without an investigation or product risk assessment for the BET result that was out of specification (OOS).
We reviewed your firm’s response and conclude it is not adequate.
Your response indicated that NC 23-042 was initiated during the inspection when the above Durepair OOS result was brought to your attention. On May 30, 2023, your firm determined the above OOS result was the result of a transcription error and the 9.98 EU result was for a different lot of product where this was an acceptable result (specification was (b)(4)).
Your response indicated that CAPA 23-026 was opened to address the above nonconformance deficiencies, yet you have not provided a copy of the CAPA nor have you identified all necessary corrective actions to demonstrate that your quality system will ensure controls are in place to prevent the release of non-conforming product. We remain concerned that your quality system will be capable of identifying similar OOS errors when you resume operations and will prevent the release of non-conforming products.
Was a Whistleblower involved?
2. Failure to establish and maintain procedures for implementing corrective and preventive actions, including requirements for analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned product, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems, as required by 21 CFR 820.100(a)(1). Specifically:
• On October 20, 2022, your firm received an internal complaint that alleged quality issues in (b)(4) manufacturing areas including your (b)(4) inspection process, bacterial endotoxin testing, bovine hide/skin thickness measurements, and control of sterilized devices.
As the result of your initial investigation, your firm initiated a production hold on December 14, 2022 and also placed (b)(4) lots of product on hold. You initiated a CAPA to address the (b)(4) issue, however, CAPAs were not opened to address the remaining (b)(4) areas of concern, including endotoxin issues.
Your CAPA procedure, GSOP-758, rev 005, indicates that “CAPA investigations and any corrections, corrective and/ or preventive actions should be (b)(4)”.
• During the inspection, you initiated a health hazard evaluation, 2023-HHE-005 to address the endotoxin concerns and the potential health risk.
This HHE, completed on April 12, 2023, was not comprehensive and did not include all complaints that may have been associated with this issue. For example, two complaints of meningitis, complaint #’s 198786 and 198790, associated with your Durepair product were not included in your initial HHE assessment that concluded a field action was not recommended. Your HHE SOP, GSOP-908, rev 015 indicates that the criteria for a HHE initiation would be when “(b)(4)”.
• Your firm failed to (b)(4) as required per your procedure, GSOP-801 rev 3, (b)(4); Rev 03. On December 14, 2022, a hold was placed on (b)(4) lots identified in the complaint but failed to identify the potential impact on (b)(4) lots manufactured during the same time frame.
The letter goes on
3. Failure to validate with a high degree of assurance, a process whose results cannot be fully verified by subsequent inspection and test, as required by 21 CFR 820.75(a). Specifically:
• Your firm failed to adequately validate your test method for testing bacterial endotoxin (BET) of finished medical devices, or the (b)(4) used during the manufacture of these devices. Review of your BET test method validation, TMVR-009; revealed the following:
o The TMVR-009 assay reports failed to meet (b)(4) testing.
(b)(4), dated January 28, 2019, excluded (b)(4) with “Sample Descriptions” of “Negative Control”, invalidating BET test method verification runs for product lots # (b)(4), and (b)(4).
(b)(4), dated January 2, 2019, excluded (b)(4) with sample descriptions “Negative Control”, invalidating BET test method verification runs for product lots # (b)(4), and (b)(4).
(b)(4), dated January 29, 2019, excluded (b)(4) with lot sample description “(b)(4)”, “Sample (b)(4)”, invalidating the BET test method verification for product lot # (b)(4).
o Your BET test method protocol, section 7.4 Execution and Documentation of BET Assay, cannot be followed as written, to accurately (b)(4) for BET analysis of device product testing. For example, Section 7.4, step 4., states, “(b)(4).” However, the referenced table lists the (b)(4).
o During routine BET testing, your firm introduced a (b)(4), in calculating the Endotoxin Limit which may cause the BET test method to be performed at (b)(4) to detect bacterial endotoxins. The use of this (b)(4) was not included in your endotoxin test method validation, TMVR-009.
Very serious threat from FDA:
Your firm should take prompt action to address any violations identified in this letter. Failure to adequately address this matter may result in regulatory action being initiated by the FDA without further notice. These actions include, but are not limited to, seizure, injunction, and civil money penalties.
Compare with Pfizer Jab Endotoxin level
The bacterial endotoxin (BET) result of 9.98 EU/Device is about half the amount found by Endotoxin expert Kevin McKernan in 1 ml of Pfizer Covid19 jab.
http://www.liderasurgical.com.br/produtos/cranio/biologicos/substituto-de-dura-mater-durepair/
https://apps.tga.gov.au/PROD/SARA/arn-detail.aspx?k=RC-2023-RN-00538-1
Joseph Matrisciano, Jr. FDA to Mr. Jan De Witte President and CEO Integra LifeSciences Corporation 1100 Campus Road Princeton, NJ. 17 July 2023.
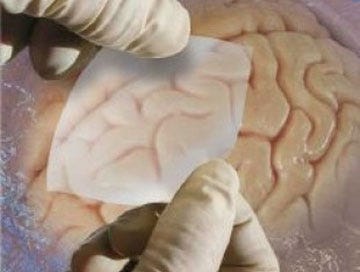


The rules don't apply to "the golden egg" products apparently.