Massive Endotoxin Contamination introduced on move from Process 1 to Process 2 mRNA
Pfizer lied to EMA for its Emergency Use Authorization claiming it could control Endotoxin in industrial scale-up. Nobel Prize winner Drew Weissman and his team of 32 helpers failed to clean it up.
Many moons ago I reported what we could find in Pfizer documents released under Court Order, or leaked, on the filthy E. coli production of jabs that were not tested on more than 250 people before Billions of jabbed people suffered the Endotoxin consequences.12
I have previously referred to work performed at Duke University by Nobel Prize winner Drew Weissman3 on the problems involved but my friend Chris Edwards prompted me to write about one paper4 5 that was published in 2021 that openly discussed the Endotoxin problem.
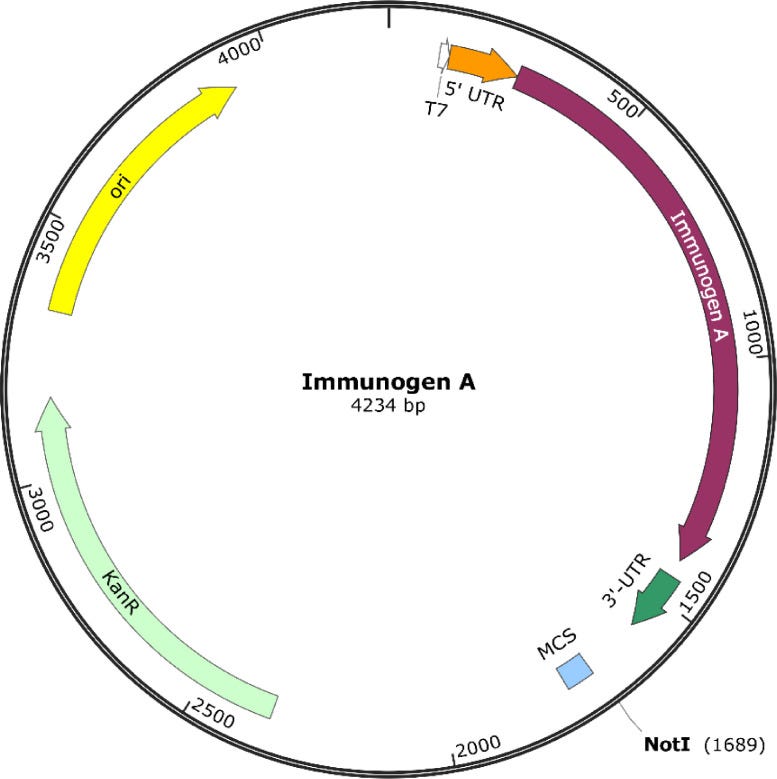
Here is the Plasmid they made.
Readers who have been following Kevin McKernan will be familiar with this type of figure. Note the transcriptional point of origin (ori in Yellow), the Kanamycin Resistance Gene (KanR in Green) and the sequence needed to generate the mRNA (Immunogen A, in Purple).
You can read the full paper or download online, but here I just highlight the fact that they mention Endotoxin 13 times.
We found that endotoxin control was the primary raw material risk due to its variability in raw material lots and the difficulty of its removal in subsequent downstream purification steps. Endotoxins can be introduced to the product primarily from IVT reaction components and large volume buffers used in purification.
and
Additional controls, such as release testing of incoming materials to detect endotoxin, vendor testing for nucleases, and sequencing to verify material prior to use are a few examples of outcomes derived and implemented from the risk assessments.
and in Table II which shows the 300ml scale results
and on “meeting regulatory expectations”
As with more established therapeutic modalities, the testing panel includes evaluation of quality attributes such as appearance, pH and osmolality, and safety tests for measuring endotoxin, bioburden and sterility.
On their Influenza jab safety for sacrificed Animals
For each antibody sequence to be tested, the mRNA heavy and light chain sequences were mixed at a 1:1 molar ratio, encapsulated into lipid nanoparticles by Acuitas Therapeutics (Vancouver, BC) and tested for acceptable endotoxin levels for testing in mice and non–human primates (NHP).
On moving to Human Jabbees
In our take on this approach, the impact to patient safety, operator safety, product quality, and regulatory hurdles can be assessed for each new material, along with adding additional controls such as release testing of incoming materials to detect endotoxin, vendor-based testing for RNase, and sequencing to verify materials prior to use.
Use of Endotoxin Removal Buffer and Chromatography
Plasmid production
The DNA plasmid is manufactured and purified by a commercial manufacturer. E. coli cells are transfected with 1–5 ng of cDNA plasmid. Transformed cells are inoculated into animal-derived component free growth media containing kanamycin. Following fermentation at 30°C for approximately 16 hours, bacterial cells are harvested by centrifugation. The cell paste is subjected to alkaline lysis with RNase A. The cell lysate is then clarified by centrifugation and adjusted to chromatography loading conditions through the addition of endotoxin removal buffer.
They saw massive increase of Endotoxin from 0.036 EU/ml, through 0.5 EU/ml, finally getting ≤30 EU/mg - note change of unit from ml to mg, for Plasmid release in their Table V.
Note that they fail to report Endotoxin measurements in Table VIII for mRNA drug substance, or Table IX for Release testing for mRNA-LNP drug product.
We know the LNP materials introduce additional Endotoxin.6
Production of the Pfizer BioNTech mRNA jabs
It seems that Fifth Column operatives want to hide some of the facts about the massive taxpayer expenditure on the failed Covid19 jabs.
Women suffer more from Pfizer Endotoxin
In 2014 Ugur Sahin and Özlem Türeci, co-founders of BioNTech, along with their colleague Katalin Karikó, mentioned the problem of Endotoxin as a contaminant in their planned mRNA jabs.
Frame-shifting Warning in 2014 from Nobel Prizer winner Katalin Karikó
No surprises anymore in the greatest disaster deliberately injected into the arms of Billions. The paper by Mulroney and coworkers is unusual because its publication was obviously delayed (Received 25 January 2023, Accepted 31 October 2023, Published 06 December 2023).
Download pdf. Jill Whitley, Christopher Zwolinski, Christian Denis, Maureen Maughan, Leonie Hayles, David Clarke, Meghan Snare, Hong Liao, Sean Chiou, Tina Marmura, Holly Zoeller, Ben Hudson, John Peart, Monica Johnson, Amelia Karlsson, Yunfei Wang, Cynthia Nagle, Cherell Harris, Daniel Tonkin, Stephanie Fraser, Lieza Capiz, Christina L Zeno, Yvonne Meli, Diana Martik, Daniel A Ozaki, Amy Caparoni, Jason E Dickens, Drew Weissman, Kevin O Saunders, Barton F Haynes, Gregory D Sempowski, Thomas N Denny, Matthew R Johnson. 2021. Translational Research. 242:38–55. https://www.translationalres.com/article/S1931-5244(21)00282-6/fulltext
Read online. https://pmc.ncbi.nlm.nih.gov/articles/PMC8641981/
Moderna ordered to get its Endotoxin Act together
Despite heavy redactions, including specifications and contamination limits, we learn much from reading the thousands of pages obtained by citizens in Freedom Of Information demands as well as court ordered releases.








Hi Geoff, Do you have a Substack article introducing readers to endotoxins? I had no clear idea what they were, other than that they were bad, but a quick look at: the page Wikipedia selects for a search for "endotoxin": https://en.wikipedia.org/wiki/Lipopolysaccharide set me on the right path.
I now understand that in the so-called "vaccine" manufacturing process used for the billions of Pfizer (Moderna too?) COVID-19 injections, the E. coli cells which are programmed, with plasmids (circular DNA), to produce the mRNA (or is it to produce DNA which is transformed into mRNA?) are naturally coated - "festooned" comes to mind - with a very large number of these lipopolysaccharide molecules on the outside of their plasma membrane. After the cells are lysed (mechanically and chemically broken apart) I assume that these endotoxin lipopolysaccharide molecules float around in the resulting fluid, probably detached from the fragments of plasma membrane, which would rapidly fall apart.
Since these molecules elicit a strong immune response in humans, to the point of causing septic shock, and since they stick onto things, such as the lipid nanoparticles which are created later in the so-called "vaccine" production process, there are obvious dangers in this manufacturing process.