Chlorine Dioxide - How does it Kill ?
Sold as a "miracle cure" this disinfectant bleach is used in many water supplies. Research is ongoing into how it kills E. coli and other life, including Humans.
A have written a little12 about Chlorine Dioxide (ClO2), the light sensitive unstable free radical, but I see from friends’ sharing that there is a thirst for science.
I found a PhD thesis written by Mischa Jütte of Darmstadt, Germany.
Here is his graphical abstract comparing destruction of E. coli bacteria with Ozone and Chlorine Dioxide.
The 252 page thesis contains lots of useful information.
He has a profile at ResearchGate is you want to follow his publications.3
His key findings were formation of Hypochlorous acid (HOCl)4 and Cl2 (FAC = Free Available Chlorine) in addition to previously known Chlorite (ClO2-).
He performed novel experiments using various substrates including phenols as models for different parts of living tissue cell surfaces and internal components including DNA.
Reactions at Stomach pH
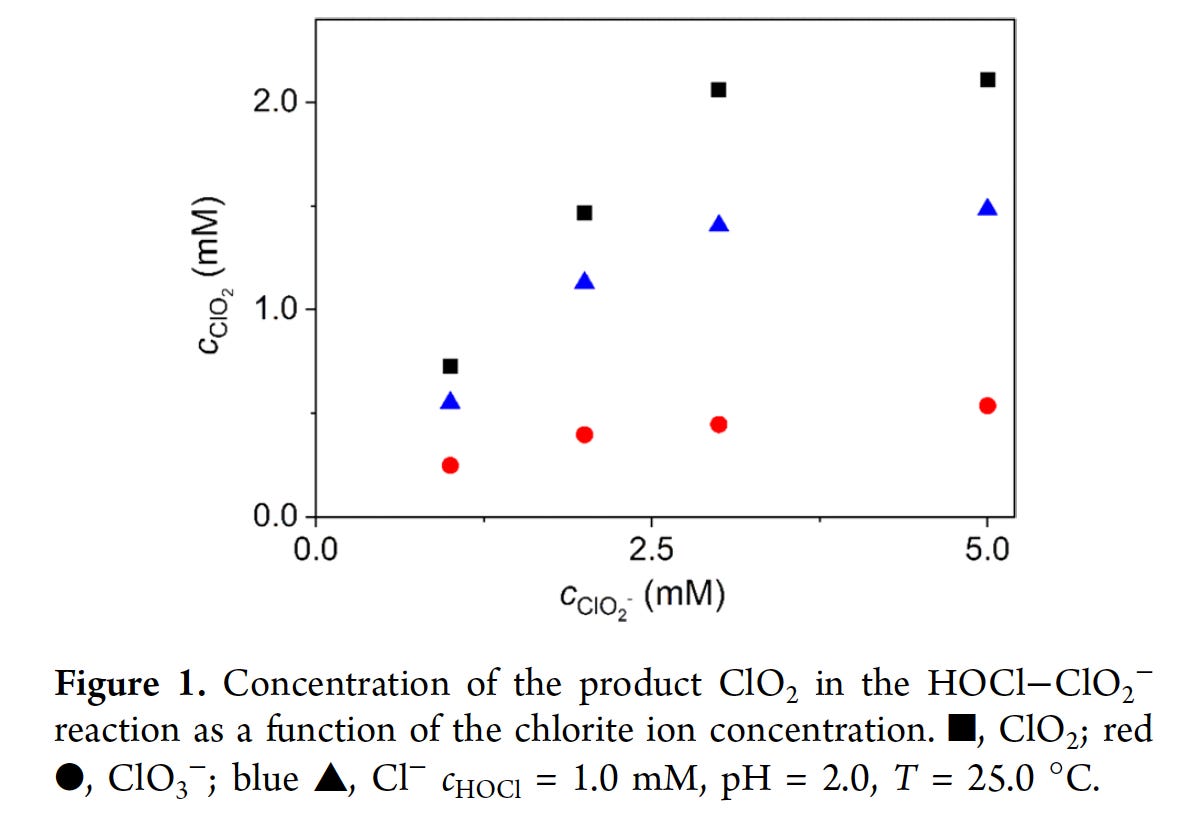
Another paper5 I found recently studied various processes including formation of ClO2, Chlorate (ClO3-)6 and Chloride (Cl-) as shown in this Figure at pH 2.
Angyal and coworkers identified 2 key intermediate species: Cl2O and Cl2O2 as shown in their abstract.
The intimate details of the reaction are explored by simultaneously fitting 78 kinetic traces, i.e., the concentration vs. time profiles of ClO2−, HOCl, and ClO2, to an 11-step kinetic model.
The most important reaction steps were identified, and it was shown that two reactive intermediates have a pivotal role in the mechanism.
While Chlorate ion predominantly forms via the reaction of Cl2O, Chlorine Dioxide is exclusively produced in reaction steps involving Cl2O2.
This study leads to clear conclusions on how to control the stoichiometry of the reaction and achieve optimum conditions to produce Chlorine Dioxide and to reduce the formation of the toxic Chlorate ion in practical applications.
Radioactive Tracer Study
There are many useful papers on what happens in solution under controlled conditions but few on the interactions of Chlorine Dioxide and its byproducts with animal tissues.
In 1982 Abdel-Rahman and coworkers used 36ClO2 given orally to Rats to see what happens.7
The primary products resulting from Cl02 disinfection of waters are Chlorites (ClO2-) and Chlorates (Cl03-).
Studies in Rats revealed that C102 is converted to chloride (Cl-), ClO2- and ClO3-.
C1O2- and ClO3- are excreted as Cl-, C102- and Cl-, C102-, Cl03-, respectively. Radioactivity was rapidly absorbed from the gastrointestinal tract following the administration of ClO2 orally, and the half-life for the elimination of 36Cl from the rat was 44 hr, corresponding to a rate constant of 0.016/hr.
After 72 hr, radioactivity was highest in plasma, followed by kidney, lung, and stomach.
36Cl in plasma reached a peak at 2 hr and 1 hr after oral administration of 36C102- and 36CI03-, respectively.
36Cl excretion was greatest 24 hr after the administration of 36Cl03-, but in the case of 36Cl02-, the excretion probably represented saturation of the biotransformation and excretion pathway. A low activity in packed cells compared to plasma was detected in Chlorate ingestion, rather than an even distribution in Chlorite treatment. Chloroform determinations in rat blood after one year indicated that Chloroform was significantly higher than control in the 100 and 1000 mg/l. C102 groups. However, no significant values were observed in the 1 or 10 mg/I. C102 and C102 metabolites group.
C102 and its metabolites are eliminated from the body more rapidly than chlorine, and they do not appear to increase trihalomethane concentrations at low dosages.
Note carcinogenic Chloroform was found.8
Here is their Table 2 showing the 36Cl radioactivity9 distribution in Rats after swallowing Chlorine Dioxide in water.
Chlorine Dioxide generated by reaction of Acid and Sodium Chlorite - FAERS update
Much discussion of mad “cures” claimed for swallowed Bleach recently.
Chlorine Dioxide Deaths and Injuries
As a child chemist, I read about the free radical Chlorine Dioxide before cautiously making it in a well ventilated area - outside in the garden.
https://www.researchgate.net/profile/Mischa-Juette
https://en.wikipedia.org/wiki/Hypochlorous_acid
Dávid Angyal, István Fábián, and Mária Szabó. 2023. Kinetic Role of Reactive Intermediates in Controlling the Formation of Chlorine Dioxide in the Hypochlorous Acid−Chlorite Ion Reaction. Inorg. Chem. 2023, 62, 5426−5434. https://doi.org/10.1021/acs.inorgchem.2c04329
https://en.wikipedia.org/wiki/Chlorate
Mohamed S. Abdel-Rahman, Daniel Couri and Richard J. Bull. 1982. Metabolism and Pharmacokinetics of Alternate Drinking Water Disinfectants. Environmental Health Perspectives 46:19-23.
https://en.wikipedia.org/wiki/Chloroform
https://en.wikipedia.org/wiki/Chlorine-36