LNPs Contaminated with Endotoxin?
Experiments on Toxicology of Lipid Nanoparticles are at risk of contamination with Endotoxin, as found for numerous flawed "Spikeopathy" studies.
Recently I updated my article on the Spike Protein derived from the Covid19 virus or the synthetic GMO Spike produced by the mRNA jabs to discuss a Zebrafish study by Zheng and coworkers that has been made invalid by Endotoxin contamination of commercial Spike.
SARS-CoV-2 Spike protein is Not pro-inflammatory in Human Primary Macrophages?
While there is no doubt the Synthetic SARS-CoV-2 Spike protein manufactured in jabbee cells, coded by synthetic mRNA in Covid19 jabs causes immense long-term damage to a generation, it can’t be responsible for the Adverse and sometimes Fatal reactions experienced in
Cationic Lipid Study in jeopardy
A recent paper1 investigating toxic effects of Lipid Nanoparticles (LNPs) used a number of Research Grade chemicals supplied by Avanti Polar Lipids.
I decided to investigate because I was aware that this company, partnered with Croda, supplied the lipids used to make the Pfizer jabs.2
I found this announcement3 on their website:
They say “Avanti does not test for mycoplasma, fungi or endotoxin in any of our products. Typically, we do not have issues with endotoxin contamination, but we cannot guarantee that of our research products will be mycoplasma, fungi or endotoxin free or sterile. However, Avanti can test for fungi or endotoxin levels and report on a CoA for any research products. If you are interested in this option, our QC group can provide pricing.”
I find this fascinating.
Military Stealth LNP developer Janos Szebeni found preparations made with Avanti Polar Lipids Distearoyl phosphatidylcholine (DSPC), dimyristoyl phosphatidylglycerol (DMPG), cholesterol (Chol) and a-tocopherol were always contaminated with ≤ 10 EU/ml.4
Any scientists who did not go to the extra expense of Endotoxin testing with a Certificate of Analysis (CoA) risk their reputation if they use the untested lipids.
The key cationic lipid made by Avanti and used by Serena Omo-Lamai and coworkers is very expensive without Endotoxin testing:
Mice exposed to aerosol Endotoxin
Serena Omo-Lamai and coworkers exposed some of their mice to nebulized Endotoxin (LPS) L2630 supplied by Sigma Aldrich, so that their laboratory air was at risk of Endotoxin contamination of control mice supposedly unexposed.
Chitosan LNPs inhibit LAL test
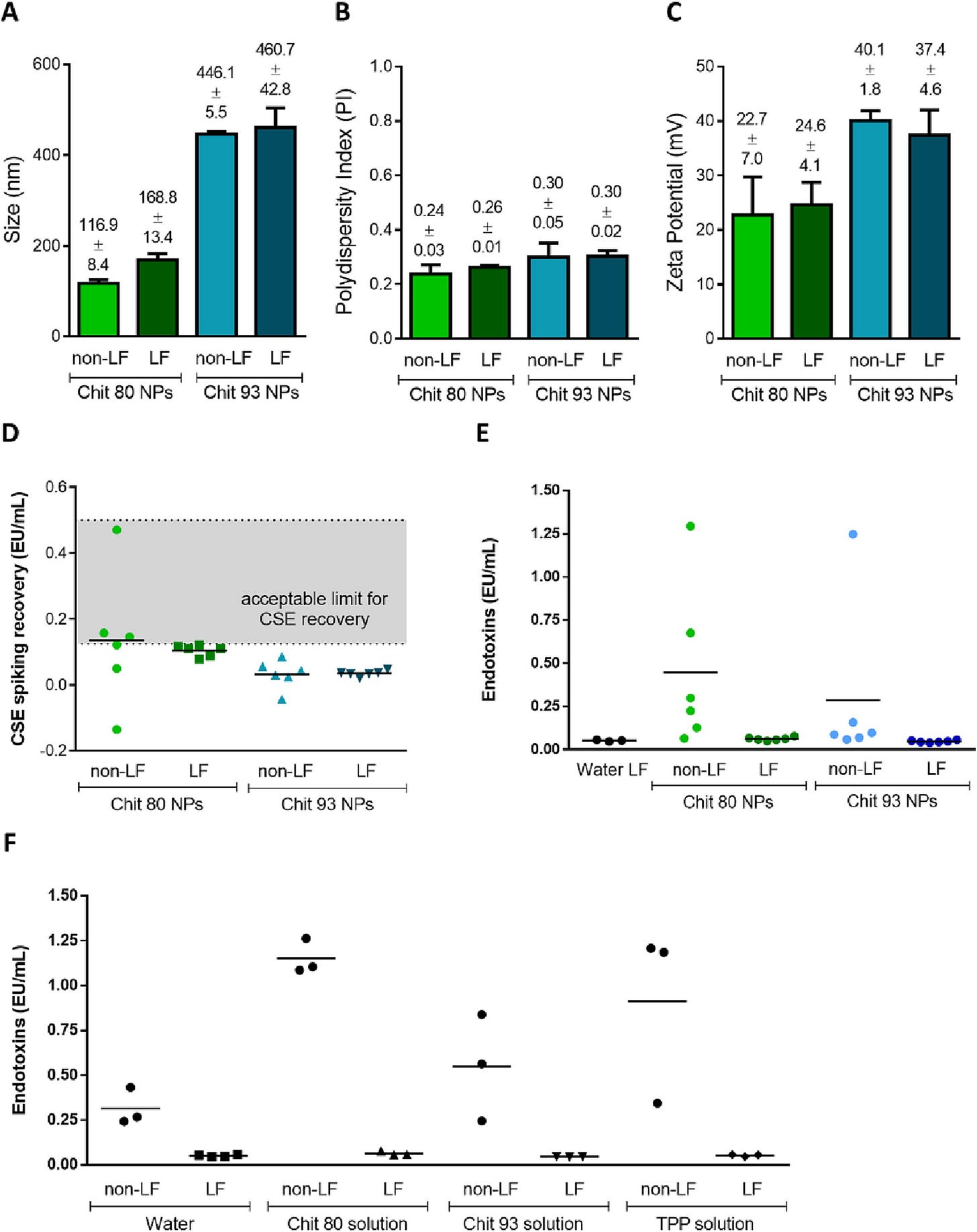
Costa and coworkers studied LNPs formed from Chitosan and found that they inhibit the LAL test.5 Interestingly they observed a reduction of LNP diameter.
What should be done?
Serena Omo-Lamai and coworkers should be encouraged to get Endotoxin analyses of all reagents used in their experiments before their preprint is reviewed for formal publication. I note they have not specified the “vehicle” used for injections either.
My earlier article reported that Endotoxin can travel on the surface and inside LNPs.
Lipid NanoParticles carry Endotoxin on their Surface as well as Inside
Recently I posted a Note which asked about Endotoxin traveling around the body from every jab from every vial of all mRNA jabs. Endotoxin upregulation of microRNA miR-155 is identified as the primary cause of Cancer induction and Metastasis by Covid19 mRNA jabs.
Serena Omo-Lamai, Marco E Zamora, Manthan N Patel, Jichuan Wu, Jia Nong, Zhicheng Wang, Alina Peshkova, Liam S Chase, Eno-Obong Essien, Vladimir Muzykantov, Oscar Marcos-Contreras, Jacob W Myerson, Jacob S Brenner. Physicochemical Targeting of Lipid Nanoparticles to the Lungs Induces Clotting: Mechanisms and Solutions. https://pubmed.ncbi.nlm.nih.gov/37546837/
Relative Lethality of COVID-19 vaccines - who is measuring the casualties?
Japan Relative Lethality data for the Covid19 Jabs This is an update to the earlier version of this article. Useful data on Total Deaths and Myocarditis Deaths after jabs by brand from Japan. Number of first and second dose by vaccine type until 14 February 2022 - Deaths - Deaths per million jabs and Myocarditis Deaths per million jabs with data to 11 Aug…
https://avantilipids.com/tech-support/faqs/mycoplasma-fungi-endotoxin-clarification
Janos Szebeni, Nabila M. Wassef, Alan S. Rudolph and Carl R. Alving. 1995. COMPLEMENT ACTNATION BY LIPOSOME-ENCAPSULATED HEMOGLOBIN IN VITRO THE ROLE OF ENDOTOXIN CONTAMINATION. Artificial Cells, Blood Substitutes, and Biotechnology. 23(3):355-363.
João Panão Costa, Sandra Jesus, Mariana Colaço, Alana Duarte, Edna Soares, Olga Borges. 2023. Endotoxin contamination of nanoparticle formulations: A concern in vaccine adjuvant mechanistic studies. https://www.sciencedirect.com/science/article/pii/S0264410X23004875