I just made a Late Comment on Australian Citizen Petition to US FDA on Covid19 Jabs
It was a little confusing, but perhaps you would like to make a submission as well?
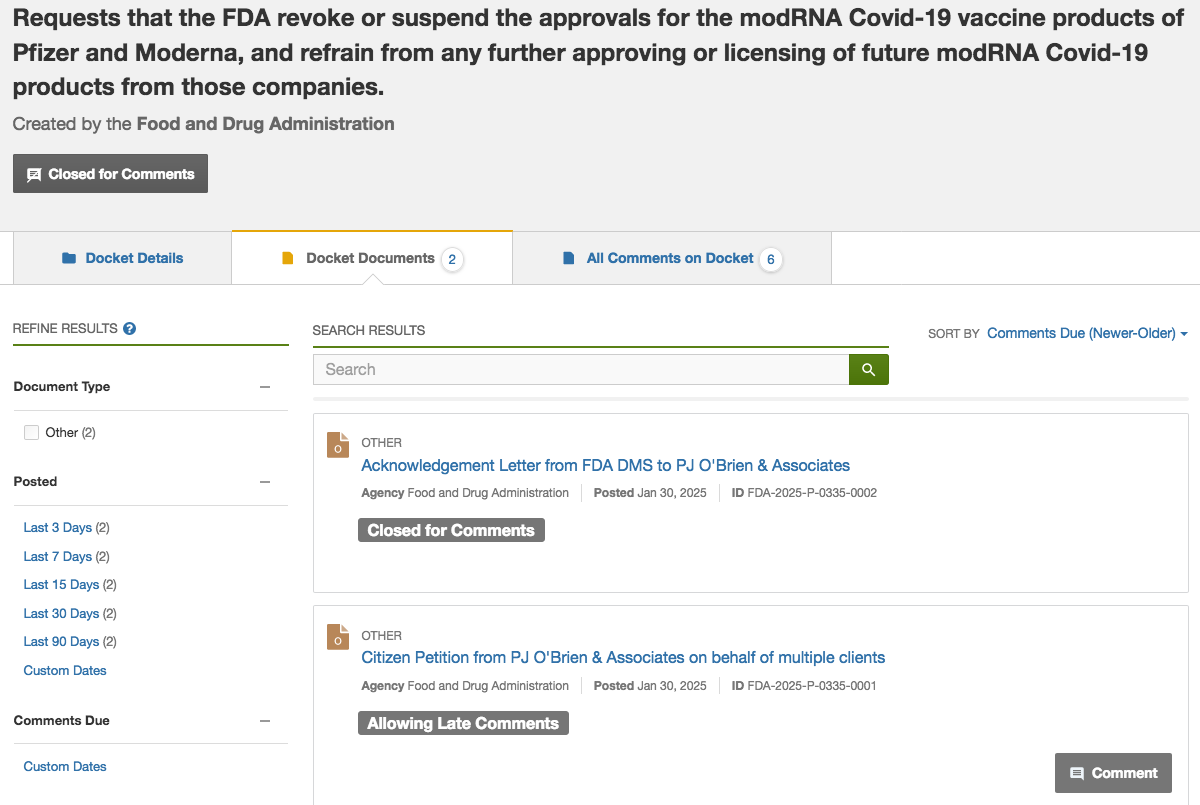
Australian Lawyers, under the banner of PJ O’Brien & Associates, have asked the FDA to “revoke or suspend” the approvals of Moderna and Pfizer mRNA Jabs, based on the failures of the FDA to refer the GMO products to the correct oversight committee - Cellular, Tissue and Gene Therapies Advisory Committee (CTGTAC) and a section of law requiring “Environmental Assessment” which includes the Human Environment.
Despite mixed messages on the Comment submission website, I lodged my comment and await the public servants’ review and decision whether to publish it.
My submission awaiting review
It took a few minutes to receive an email receipt from Regulations.gov
If approved for publication, I will have the opportunity to remove any typos.
Please click to enlarge.
You can see I concentrated on the possible incorporation of the Synthetic or Bacterial host cell DNA or RNA into the Germline cells of Humans via Endotoxin enhanced nuclear transcription processes involving Zinc Finger proteins or Lipopolysaccharide Binding Proteins.1
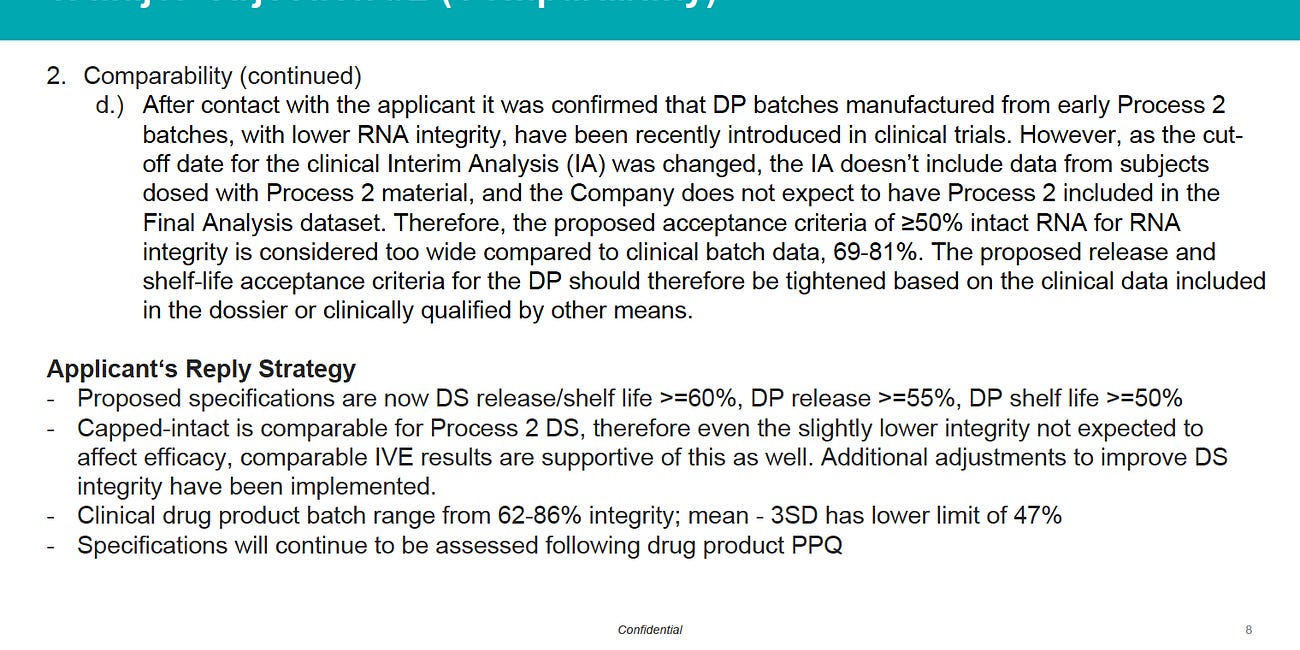
I also reminded them that Pfizer patented Endotoxin Lipid A as their preferred “adjuvant” obtained in uncontrolled amounts from the filthy Bacterial soups used to produce the “Process 2” Poojabs.2
I reminded them that FDA CBER is very worried about Endotoxin, such as their directive to Moderna in late 2021 to source Pharmaceutical grade Cholesterol for their LNPs.3
I decided to keep my submission very brief but attached a copy of the AMPS Book “Too Many Dead” to which I contributed a chapter.4
The PJ O’Brien & Associates Citizen Petition introduction reads:
The PJ O’Brien & Associates Citizen Petition does not mention Endotoxin, but I am pleased to see that one of the people who has already had their comment published, Daniel Saxelby, of Watchmaker Genomics, Spicewood, Texas, did mention Endotoxin problems detected during FDA inspection of Catalent in Bloomington Indiana.
You see in my draft comment awaiting approval that I reminded them that FDA inspections at Rentschler in Germany also found Endotoxin a major worry.
Please let me know if you add a comment to the Petition.
Yin Yang 1 requires Endotoxin to activate Germline DNA transcription and Class Switch Recombination
Gathering some more thoughts on how foreign DNA in Jabs could end up changing the Human Genome as well as causing immense suffering.
Production of the Pfizer BioNTech mRNA Poojabs
It seems that Fifth Column operatives want to hide some of the facts about the massive taxpayer expenditure on the failed Covid19 jabs.
Moderna ordered to get its Endotoxin Act together
Despite heavy redactions, including specifications and contamination limits, we learn much from reading the thousands of pages obtained by citizens in Freedom Of Information demands as well as court ordered releases.