TGA E. Coli PlasmidGate 2017
In 2017, Therapeutics Goods Administration reported on Human and Animal testing of injections of Plasmid DNA from E. coli. This might be old news to some?
Please excuse me if other Substack authors have covered this before, and point me to their articles.
I will do more searching under #PlasmidGate, much of which I missed when I was suspended from Twitter.
There is much yet to be found on GMO technology in Australia, as we prepare for the Royal Commission into Covid19, so I did another search of government websites and found this interesting item:
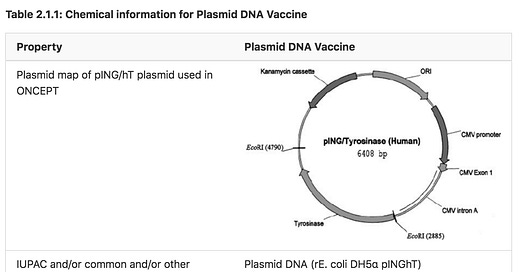
An application was submitted by the Australian Pesticides and Veterinary Medicines Authority (APVMA) to create a new entry for plasmid DNA (rE. coli DH5α pINGhT) in Schedule 4 of the Poisons Standard, with no exemption cut-off.1
Note especially the CMV Promoter and Kanamycin Cassette that expresses the gene coding for the full-length sequence of Human Tyrosinase. That is correct, you read HUMAN !!
Very interesting, thought I. Have Kevin McKernan or ArkMedic covered this?
This was a general application. The APVMA's proposed amendments to the Poisons Standard are:
Schedule 4 – New Entry
PLASMID DNA (rE. coli DH5α pINGhT). (highlighted in Bright Red)
The applicant's reasons for the request are:
The proposed new product contains a new active constituent, plasmid DNA
(rE. coli DH5α pINGhT) that expresses the gene coding for the full-length sequence of human tyrosinase. It is designed to stimulate an immune response targeted at canine melanoma cells expressing tyrosinase. It is proposed for the vaccination of dogs with Stage II or III oral melanoma, for which local disease control has been achieved, to aid the extension of survival time.
No specific toxicity studies or associated data on either the active constituent or the formulated product were submitted with the application. However, some relevant studies from published literature were submitted in response to questions from the APVMA. These studies involved the administration to both humans and other animal species, of vaccines that were the same as or similar to the proposed product. Additionally, there were studies on other relevant DNA vaccines. Data from these studies suggested that the proposed product vaccine is unlikely to be associated with any significant toxic effects in humans
The product is a therapeutic vaccine that is applied by a spring powered device that does not require the use of needles. This technology has the potential to make the administration of medicine safer as well as efficient and convenient. Post-application exposure is likely to be negligible. Any residual vaccine on the skin surface is likely to very small. The needle free device means that there are no needles that require disposal. However, the use of this canine transdermal device requires trained personnel.
As the amounts of DNA injected are small (about 110 µg), treatment is systemic (IM administration), the DNA will not replicate in the body of the injected animal or be excreted intact, no exposure is anticipated to occur during re-handling. As DNA is a normal component of the body, its breakdown products are not toxic, so urine/faeces of the injected animal will not contain hazardous substances arising from vaccination.
The applicant has requested scheduling as Schedule 4 Prescription animal remedy because of precedence of other vaccines in the Poisons Standard.
“The dosage of Plasmid DNA (rE. coli DH5α pINGhT) in the vaccine is low, 110 µg per 0.4 mL dose”
Withdrawal of Approval from EMA in 2015
Merial Oncept Melanoma application for marketing approval, lodged in 2013 was withdrawn in 2015.2
In its letter notifying the Agency of the withdrawal of application, the applicant stated the reason for the withdrawal: The company's additional investment in research and development required to answer the remaining issues is not justified.
Marketing in Australia
Boehringer Ingelheim Animal Health Australia Pty. Ltd. who purchased Merial.3
Efficacy Questioned
Not a glowing endorsement.4
Merial involved in Covid19 and Flu jabs
A jab involving Beijing Merial Vital Laboratory Animal Technology, who propagated Bird Flu Virus H1N1 A/California/07/2009 in specific pathogen-free (SPF) embryonated eggs, has been reported with up to 2.5 EU/ml Endotoxin.5
October 2024 Update
TGA uses PCR to amplify Kanamycin Resistance Gene to measure Residual Plasmid DNA in Moderna Covid19 Jabs. See FOI 5286.6
Australian taxpayers cough up $50 per sample just for the Kanamycin Resistance Gene Residual DNA test for each Batch Release.7
https://www.tga.gov.au/resources/publication/scheduling-decisions-interim/scheduling-delegates-interim-decisions-and-invitation-further-comment-accsacms-march-and-july-2017/21-plasmid-dna-vaccine
https://www.ema.europa.eu/en/medicines/veterinary/withdrawn-applications/oncept-melanoma
https://en.wikipedia.org/wiki/Boehringer_Ingelheim_Animal_Health
Pellin MA. 2022. The Use of Oncept Melanoma Vaccine in Veterinary Patients: A Review of the Literature. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9693055/
Shi R, et al. 2022. A combination vaccine against SARS-CoV-2 and H1N1 influenza based on receptor binding domain trimerized by six-helix bundle fusion core. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(22)00479-0/fulltext
TGA FOI 5286. Protocol for assessing the presence of residual DNA in the mRNA COVID-19 vaccine products provisionally approved by the TGA. 19 September 2024. https://www.tga.gov.au/foi-disclosure-log
https://www.thermofisher.com/order/catalog/product/A50337