Pfizer BioNTech Covid19 Jab Multiple Trials Trail
Murky world of jabs trials, published and unpublished by BioNTech and Pfizer
As many authors have discussed the failure of the BioNTech Pfizer Covid19 jabs and the massive casualties, this post is a quick guide to the multiple Phase 1/2/3 trials they launched around the world involving tens of thousands of recruited volunteers.
If you find it confusing, you are not alone. Here is a page outlining the trials within trials that was recently shared by Sonia Elijah, obtained by FOI demand.1
53,499 trial subjects recruited to 18 June 2021.
German BioNTech trial
As mentioned in my previous article, the German Non-Randomized arm of the trial2 conducted in 5 locations, showed that the free floating BNT162b1 spike caused increased C-Reactive Protein and massive collapse of Lympocytes.3
The German trial had two parts, using 512 volunteers, (some of whom were paid) in 5 locations, Part A and Part B. Due to changes in the overall clinical development plan, Part B was abandoned.
It used four different experimental jabs: BNT162a1, BNT162b1, BNT162b2, and BNT162c2
ClinicalTrials.gov Identifier: NCT04380701
Other Study ID Numbers: BNT162-01; 2020-001038-36 ( EudraCT Number )
U1111-1249-4220 ( Other Identifier: WHO UTN )
Start Date: 23 April 2020
Planned Finish Date: April 2023
Canceled: 18 January 2023
No plan to share Individual Participant Data (IPD) of 512 recruits.
Funded by BioNTech.
Results have not been posted to the US Clinical Trials website.
A publication makes reference to this trial.4
China Trial
This trial was primarily designed to study racial effects of the subsequently abandoned BNT162b1 jab, one of the four initial Drug Substances evaluated.5
ClinicalTrials.gov Identifier: NCT04523571
Other Study ID Numbers: BNT162-03
Started 28 July 2020, ended 10 August 2021.
No plan to share Individual Participant Data (IPD) of 144 recruits 18 Years to 85 Years old.
Funded by BioNTech in collaboration with Shanghai Fosun Pharmaceutical Industrial Development Co. Ltd.
Results published in 2022.6
Greek Trial
ClinicalTrials.gov Identifier: NCT04743388
Started 4 Jan 2021, Ended? Dec 20227
Kinetics of antibodies against SARS-CoV-2 as well as the kinetics of the immune system's cell subpopulations and cytokines associated with the immune system in healthy volunteers receiving the BNT162b2 vaccine against SARS-Cov-2 of the Pfizer/BioNTech companies or any other vaccine authorized and administered by the Ministry of Health.
Publication of Inflammatory Cytokines and Chemokines after Jabs 1 and 2.8
Funded by US NIH.
Largest Trial
The most talked about trial conducted in 168 locations in Argentina, Brazil, Germany, South Africa, Turkey, USA. Described as “Phase 1/2/3, randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in healthy individuals.” It initially used BNT162b1 and BNT162b2.9
Actual enrolment: 45713 participants.
It is ongoing to include assessment of boostability in a subset of Phase 3 participants at selected sites in the US who will receive a third dose of BNT162b2 at 30 µg or a third and potentially a fourth dose of prototype BNT162b2VOC at 30 µg of BNT162b2s01, based upon the South African variant and now referred to as BNT162b2SA. A further subset of Phase 3 participants will receive a third, lower, dose of BNT162b2 at 5 or 10 µg.
Responsible Party: BioNTech with Pfizer as co-sponsor.
ClinicalTrials.gov Identifier: NCT04368728
Other Study ID Numbers: C4591001; 2020-002641-42 ( EudraCT Number )
Started 29 April 2020, planned to finish 24 March 2023, when Emergency Use Authorization will cease.
A number of publications have arisen from this trial.10
Jabbing Children aged 12 to 15
A subset of the NCT04368728 involved recruitment of 2,264 children and results were published.11 A substantial thread12 discussing details of this jabbing was posted on Twitter by Jonathan Weissman13 before my account was suspended, but carrying through during the period while I suspended. It was brought to my attention on 14 April 2023, by my good friend OpenVAET, prompting me to update this Substack.
His key findings are summarized here.
1180 of the Children received at least one 30μg dose before the data cutoff date, 13 March 2021, comprised of 1131 adolescents during the blinded study and 49 from the Placebo group, who turned 16, were unblinded and chose to be treated.
Adverse Effects included 1 related Life-threatening Fever, 1 Life-threatening Anaphylaxis, 1 case of Myopericarditis, hospitalised, and 3 Children on SSRI medication for Depression, each hospitalised with symptom “exacerbation”.
Victims of this jabbing trial included Maddie de Garay, who suffered Severe Abdominal/chest pain and Extreme Numbness within a day of dose 2. She developed blood in her urine, mobility issues, is now paralysed and uses a nasogastric tube to eat. Mislabelled as “functional abdominal pain” and “neuralgia”.
Further, Jonathan Weissmann found Lymphadenopathy, swollen lymph nodes, occurred at a statistically significantly higher rate in the treatment group (9 vs. 2 in Placebo). The severe Fever rate after the first Jab obviously prompted the administrator of Dose 2 to take preemptive action, ensuring in the treatment group, 51% used antipyretic medication vs. 9% in the Placebo group.
Here I place Jonathan Weissman’s summary Table, for those who don’t want to visit the Twitter thread.
Lyophilized Formulation Trial
BioNTech investigated injection with freeze dried jab.14
This Phase 3 trial recruited 629 participants aged 18-55 Years using the frozen-liquid formulation, offered to participants who originally received 2 doses of the lyophilized formulation of BNT162b2. The trial was conducted at 21 locations, exclusively in USA.
ClinicalTrials.gov Identifier: NCT04816669
Other Study ID Numbers: C4591020
This trial has an extended list of Adverse Events.
Started 1 April 2021, ended 2 December 2021.
Jabbing Children aged 6 months to 18 years
Responsible Party: BioNTech SE
ClinicalTrials.gov Identifier: NCT0481664315
Other Study ID Numbers: C4591007; 2020-005442-42 ( EudraCT Number )
No plan to share Individual Participant Data (IPD) of 11,111 recruits.
Funded by BioNTech and Pfizer.
135 Study Locations in Brazil, Finland, Mexico, Poland, Spain, USA.
Open label.
Doses ranged from 3 to 30 migrogam of RNA BNT162b2.
Heart damage recorded by Troponin testing in adolescents.
Started 24 March 2021, completion 3 October 2024.
Results published in NEJM.16
Acute post-Jab Myocarditis C4591036
Using primary data from the Pediatric Heart Network (PHN), a NIH funded consortium of hospitals to characterize the clinical course, risk factors, long-term sequelae, and quality of life in children and young adults <21 years with Acute post-vaccine Myocarditis over a 5 year period.
Update April 2024.
The Myocarditis study NCT05295290 deadline has been extended to 21 November 2030.17
Japanese Jabbees
Phase 1/2, randomized, placebo-controlled, and observer-blind study in healthy Japanese adults aged 20-85, excluding those who were suicidal.
ClinicalTrials.gov Identifier: NCT04588480
Other Study ID Number: C4591005
No plan to share Individual Participant Data (IPD) of 160 recruits.
Funded by BioNTech and Pfizer.
Product Manufactured in and Exported from the US.
Started 21 Oct 2020, finished 25 Nov 2021.
Results published in Nature Communications with authors shareholdings identified.18
Japanese Healthcare Workers C4591006
Study Title: General Investigation of Comirnaty Intramuscular Injection (Follow-up study for Subjects [Healthcare Professionals] Who are Vaccinated at an Early post- Approval Stage)
Country: Japan
Study objective: The healthcare professionals who are vaccinated with this product early after the marketing approval of this product (participants in the Investigation of Health Status of Recipients Vaccinated First conducted by the Science Research Group of the Ministry of Health, Labour and Welfare) will be followed for 11 months from the day following 28 days after the final vaccination of this product (end date of observation period in Investigation of Health Status of Recipients Vaccinated First) to 12 months after the final vaccination of this product, information on serious adverse events and COVID-19 observed during the follow-up period will be collected, and the long-term safety of this product will be assessed (to be conducted as 11-month follow-up investigation after completion of Investigation of Health Status of Recipients Vaccinated First).
Jabbing Pregnant Women
No plan to share Individual Participant Data (IPD) of 389 recruits.
ClinicalTrials.gov Identifier: NCT04754594
Other Study ID Number: C4591015; 2020-005444-35 ( EudraCT Number )
Funded by BioNTech and Pfizer.
Product Manufactured in and Exported from the US.
Started 16 February 2021, finished 15 July 2022.
Results are now published 7 August 202319, previously discussed elsewhere.2021
86 Study Locations in Brazil, South Africa, Spain, UK, USA.
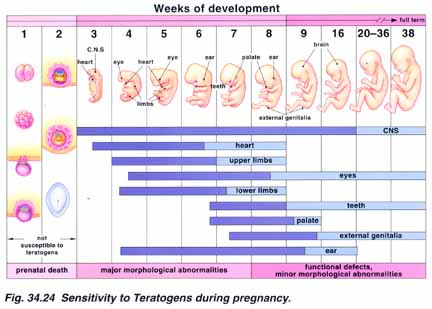
Significantly higher Birth Defects are reported in Children of Jabbed Mothers, which will no doubt be covered in the Teratology study ongoing. These included Atrial septal defect, Congenital naevus, DiGeorge's syndrome, Hydrocele, Hypotonia, Microcephaly, Mucopolysaccharidosis, Polydactyly, Syndactyly, Trisomy 21, Jaundice, Low birth weight baby, Small for dates baby, Meconium aspiration syndrome, Apgar score low,
Pregnancy Outcomes in Pfizer Trials
Recently released document shows tragic consequences of Pfizer Jab Trials using 149 Pregnant Women and their non-consenting Foetuses in US, Argentina, Brazil, South Africa, Germany and Turkey. 30 had Placebo.
Note they combine results from Trials C45911001, C4591015, BNT162-01, C4591017, BNT162b2 and the mysterious “Blinded Therapy”.
17 Spontaneous Abortions, Abortion Incomplete (2), Hyperemesis Gravidum, Miscarriage of Partner, Pre-Eclampsia, Premature Separation of Placenta, Retained Products of Conception, Vaginal Haemorrhage, Dyspneoea, Pruritis.
6 Serious Baby Cases (3 exposed 3rd Trimester), 4 babies had Deformities, Hypoxic-Ischaemic Encephelopathy, Neonatal Respiratory Failure, Shock, Intestinal Perforation, damaged Foetal Heart Rate pattern, Pneumoperitoneum, Renal Tubular Necrosis, Acidosis, Pneumothorax.
9 Elective Terminations.
Non-Interventional Trial C4591009
What happens to people who were never jabbed versus the Billions who rolled up their sleeves? Pfizer is quietly looking at that in their “Non-Interventional Trial” C4591009.22 The US FDA insisted on this study as shown on this page that was shared23 on Twitter while I was suspended:
Note the US FDA extreme concern (muffled) demanding Myocarditis and Pericarditis be studied with a reporting date of 31 October 2025.
Pfizer followed FDA orders and created the Protocol.
Research question and objectives:
Research question: What are the incidence rates/prevalence of safety events of interest among individuals vaccinated with the Pfizer-BioNTech COVID-19 vaccine within selected United States data sources participating in the Sentinel System, overall and in subpopulations of interest (pregnant women, immunocompromised individuals, and individuals with a history of COVID-19) compared with rates of those events in individuals who have not received any vaccination for COVID-19?
One of the recently obtained documents from Pfizer runs to 393 pages of Adverse Events and in the Tables we see, without discussion on the right hand columns the data for the C4591009 study using the worldwide resources of Pfizer.
Here is one page the Non-Interventional Study that covers casualties to 21 June 2022.
New Myocarditis cases reported at the rate of 2,312 per month for Jabbees versus 7 per month for the Unjabbed
Teratology Trial C4591022
Teratology is the scientific study of Congenital Abnormalities and Abnormal Formations
Various organ of the developing foetus are more vulnerable at various stages of a pregnancy with prenatal Death most common within 2 weeks of conception.
Study C4591022, entitled “Pfizer-BioNTech COVID-19 Vaccine Exposure during Pregnancy: A Non-Interventional Post-Approval Safety Study of Pregnancy and Infant Outcomes in the Organization of Teratology Information Specialists (OTIS)/MotherToBaby Pregnancy Registry.”24
Final Protocol Submission: July 1, 2021
Study Completion: June 30, 2025
Final Report Submission: December 31, 2025
Jabbing Immunocompromised People
Planned trial C4591018 was replaced by trial C4591024 which finally enrolled only 124 participants aged 2 years or older in 23 locations in Brazil, Germany, Mexico and USA.25
Responsible Party: BioNTech with Pfizer as co-sponsor.
ClinicalTrials.gov Identifier: NCT04895982
Other Study ID Numbers: C4591024; 2021-001290-23 ( EudraCT Number)
Started 15 October 2021, finishing 5 April 2024.
No plan to share Individual Participant Data.
Results have been posted.26
102 Children (2-11 years); 15 Adolescents (12-17 years); 5 Adults (18-64 years); 2 Adults aged 65 to 84 years.
Different doses were used according to age group.
Boosted Jabbees comparing Lots
Phase 3 trial using 2 SARS-CoV-2 RNA vaccine candidates, BNT162b2 and BNT162b2.B.1.351 with age restricted to those 12-50 years already jabbed, so no Placebo.27
No plan to share Individual Participant Data (IPD) of 1,574 recruits in US only.
Responsible Party: BioNTech SE
ClinicalTrials.gov Identifier: NCT04713553
Other Study ID Number: C4591017
Funded by BioNTech and Pfizer.
Product Manufactured in and Exported from the US (3 Lots) and Europe (1 Lot).
Started 15 February 2021, finished 15 July 2021.
Notable for Withdrawals and “Lost to Follow-up”
Resulted in 1 Spontaneous Abortion.
There IS an agreement between Principal Investigators and the Sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed.
Results available.28
Pneumonococcal together with Covid BNT162b2
This triple masked, randomized Phase 3 study in 27 locations and was restricted to jabbees 65 years and older who had received 2 doses of BNT16b2 in Trial C4591001. Actual recrutment was 570.29
They were split into 3 groups given:
1] 20-valent pneumococcal conjugate vaccine (20vPnC) and a third jab of BNT162b2 simultaneously.
2] 20vPnC or Saline Placebo
3] BNT162b or Saline Placebo
Responsible Party: Pfizer
ClinicalTrials.gov Identifier: NCT04887948
Other Study ID Number: B7471026
Funded by Pfizer.
Started 20 May 2021, finished 8 December 2021.
One patient who received both 20vPnC and BNT162b2 died.
27 people did not complete the study including 4 “Lost to Follow-up”, 5 removed bu “Physician Decision” 2 “Protocol Violations” 7 “Withdrawal by Subject” 4 “No longer met eligibility criteria” and 4 “Other”.
Apert from 1 Death, there were cases of Unstable Angina, Duodenal perforation, 5 cases of different cancers, and 1 case of Acute Kidney injury.
They plan to share Individual Participant Data (IPD) with selected researchers.
There is an agreement between Principal Investigators (PIs) and Pfizer (or its agents) that restricted the PI's rights to discuss or publish trial results after the trial was completed.
Lower Dose Trial C4591007
Study C4591007 substudy to evaluate the immunogenicity and safety of lower dose levels of COMIRNATY in individuals 12 through <30 years of age.
Final Protocol Submission: September 30, 2021
Study Completion: November 30, 2023
Final Report Submission: May 31, 2024
Severe or Atypical COVID-19 disease C4591008
Study Title: HERO Together: A post-Emergency Use Authorization observational cohort study to evaluate the safety of the Pfizer-BioNTech COVID-19 vaccine in US healthcare workers.
Country: United States
Primary study objectives:
• Estimate the real-world incidence of safety events of interest and other clinically significant events among US healthcare workers vaccinated with the Pfizer-BioNTech COVID-19 vaccine following Emergency Use Authorization.
Secondary objectives:
• Evaluate whether the vaccine recipients experience increased risk of safety events of interest and other clinically significant events post vaccination.
• Estimate the incidence rates of safety events of interest and other clinically significant events among sub cohorts of interest such as individuals who are pregnant, individuals who are immunocompromised, and stratified by age.
Assessment of occurrence of safety events of interest, including severe or atypical COVID-19 disease in real-world use ofCOVID-19 mRNA vaccine.
US Defense Department Personnel C4591011
Assessment of occurrence of safety events of interest, including severe or atypical COVID-19 disease in a cohort of people within the Department of Defense Healthcare System. Using secondary data from administrative claims/electronic medical records for military and civilian personnel and their families in the Department of Defense Military Health System.
Veterans Trial C4591012
Study C4591012, entitled “Post-emergency Use Authorization Active Safety Surveillance Study Among Individuals in the Veteran’s Affairs Health System Receiving Pfizer-BioNTech Coronavirus Disease 2019 (COVID-19) Vaccine.”
Looks like Anaphylaxis was top of list in a number or Pfizer trials, Myocarditis and Pericarditis were added as safety concerns for Veterans in September 2021.
Primary study objectives:
• To assess whether individuals in the VHA system experience increased risk of safety events of interest following receipt of the Pfizer-BioNTech COVID-19 vaccine.
• To assess whether sub-cohorts of interest (i.e., immunocompromised, elderly, individuals with specific comorbidities, individuals receiving only one dose of the Pfizer-BioNTech COVID-19 vaccine, and individuals with prior SARS-CoV-2 infection) in the VHA system experience increased risk of safety events of interest following receipt of the Pfizer-BioNTech COVID-19 vaccine.
Secondary study objective:
• To characterize utilization patterns of the Pfizer-BioNTech COVID-19 vaccine among individuals within the VHA, including estimating the proportion of individuals receiving vaccine, 2-dose vaccine completion rate, and distribution of time gaps between the first and second dose, demographics and health histories of recipients, overall and among the sub-cohorts of interest.
Final Protocol Submission: January 29, 2021
Study Completion: June 30, 2023
Final Report Submission: December 31, 2023
Within the first interim report of the PASS study C4591012, which is listed in the RMP of Comirnaty, (procedure EMEA/H/C/005735/MEA/010), only demographic and clinical characteristics of the two cohorts (Pfizer-BioNTech COVID-19 vaccine recipients and seasonal influenza vaccines recipients) were provided. No safety information was provided in the first interim report of study C4591012.
Kaiser Permanente Southern California C4591014
includes Children aged 5 to 11 ??
ClinicalTrials.gov Identifier: NCT0484858430
Study C4591014, entitled “Pfizer-BioNTech COVID-19 BNT162b2 Vaccine Effectiveness Study - Kaiser Permanente Southern California.”
Final Protocol Submission: March 22, 2021
Study Completion: December 31, 2022
Final Report Submission: June 30, 2023
Note this study protocol stated recruits would be aged 16 years or older who are admitted to the hospital (primary objective) with acute respiratory infection (ARI) after 14 December 2020 (date of first vaccinations at KPSC).
On 28 Jan 2022m the protocol was suddenly changed to include Children aged 5 or more.31
European Trial C4591038
Former C4591021 substudy. A collaboration with University Medical Center Utrecht on behalf of VAC4EU Consortium research team and is designed as a substudy of C4591021 to assess the natural history of post-vaccination Myo-/Pericarditis, e.g., recovery status (using medical record review) and/or identification of serious cardiovascular outcomes (using existing structured data) within 1 year of Myo-/Pericarditis diagnosis among occurring in individuals vaccinated with COMIRNATY as well as individuals not vaccinated with a COVID-19 vaccine.
October 2023 Update
Erika Delph of the my Daily Clout Team 3 has found the key to the “Bait and Switch” by Pfizer who have not yet released details of the 252 people who were given E. coli Process 2 jabs. Please see my earlier article, now updated, on Production for details.32
Hands up anyone inspired by the trials?
"If you have to be persuaded, reminded, pressured, lied to, incentivized, coerced, bullied, socially shamed, guilt-tripped, threatened, punished and criminalized; if all of this is considered necessary to gain your compliance – you can be absolutely certain that what is being promoted is not in your best interest." Attributed to Ian Watson.
https://clinicaltrials.gov/ct2/show/NCT04380701
Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. September 2020. https://www.nature.com/articles/s41586-020-2814-7
https://clinicaltrials.gov/ct2/show/NCT04523571
Li J, et al. 2022. Immune Persistence and Safety After SARS-CoV-2 BNT162b1 mRNA Vaccination in Chinese Adults: A Randomized, Placebo-Controlled, Double-Blind Phase 1 Trial. https://pubmed.ncbi.nlm.nih.gov/35771353/
https://www.clinicaltrials.gov/study/NCT04743388
https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00932-3
https://clinicaltrials.gov/ct2/show/NCT04368728
Walsh EE, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. https://www.nejm.org/doi/10.1056/NEJMoa2027906
Frenck RW, et al. 2021. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. https://www.nejm.org/doi/full/10.1056/nejmoa2107456
https://clinicaltrials.gov/ct2/show/NCT04816669
https://clinicaltrials.gov/ct2/show/NCT04816643
Walter EB, et al. 2021. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. https://www.nejm.org/doi/10.1056/NEJMoa2116298
Pfizer. A Study to Learn About The COVID-19 (Study) Vaccine (Called COMIRNATY) in People That Are Less Than 21 Years Old. https://www.clinicaltrials.gov/study/NCT05295290
Haranaka M, et al. 2021. A randomized study to evaluate safety and immunogenicity of the BNT162b2 COVID-19 vaccine in healthy Japanese adults. https://pubmed.ncbi.nlm.nih.gov/34907170/
https://classic.clinicaltrials.gov/ct2/history/NCT04754594?V_21
Nana M, et al. Diagnosis and management of covid-19 in pregnancy. https://www.bmj.com/content/377/bmj-2021-069739.long
Mohapatra S, et al. 2022. Pharmacological consideration of COVID-19 infection and vaccines in pregnancy. https://pubmed.ncbi.nlm.nih.gov/35316227/
Pfizer. 2021. A Non-Interventional Post-Approval Safety Study of the Pfizer-BioNTech COVID-19 mRNA Vaccine in the United States.
https://twitter.com/Megalodon_2nd/status/1588652289132109824
https://catalogues.ema.europa.eu/node/3169/administrative-details
https://clinicaltrials.gov/ct2/show/NCT04895982
https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-001290-23/results
https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-005903-11/results
https://clinicaltrials.gov/ct2/show/results/NCT04713553
https://clinicaltrials.gov/ct2/show/NCT04887948
https://clinicaltrials.gov/ct2/show/NCT04848584
https://clinicaltrials.gov/ct2/history/NCT04848584?V_6=View#StudyPageTop
Production of the Pfizer BioNTech mRNA jabs
It seems that Fifth Column operatives want to hide some of the facts about the massive taxpayer expenditure on the failed Covid19 jabs. Here I will concentrate on Pfizer BioNTech that has achieved market dominance. The production processes for materials used in the Phase 1 trial were not the same as those used in the Phase 2/3 trials, and most likely con…











Thanks Dr Geoff. This is a great reference.
The Japanese and Chinese trials both state the research is in accordance with the 1964 Helsinki Declaration.
I guess it is ironic that in reality, with the mandates and social coercion, that the Declaration was totally trod upon.
I mean, this is the first general principle stated:
" The Declaration of Geneva of the WMA binds the physician with the words, ****“The health of my patient will be my first consideration,”**** and the International Code of Medical Ethics declares that, “A physician shall act in the patient’s best interest when providing medical care.”"
Pretty sure that was NOT the first consideration these last few years, sadly.
The 8th clause:
"While the primary purpose of medical research is to generate new knowledge, this goal can never take precedence over the rights and interests of individual research subjects."
And here is the 9th clause:
" It is the duty of physicians who are involved in medical research to protect the life, health, ****dignity, integrity, right to self-determination,**** privacy, and confidentiality of personal information of research subjects. "
Probably quoting this Declaration is "disinformation" at this point. How can anyone have the "right to self-determination" when Pfizer's global expansion is at stake???
Anyway I will have to study the results of these different trials so more a bit later.
Already the Japanese one showed that 1 person in the quite small BNT162b2 group (119 people) had such a severe systemic adverse reaction that this person had to discontinue. Not a great sign but ignored totally by the world of course...
Geoff, what is your @ twitter handle that is suspended? I can't believe they reinstated me and am going to push for some others to also be freed.