No Limit to German Endotoxin Madness !
European Medicines Agency advisor wanted no Limit imposed on Endotoxin and other contaminants in Bio Therapeutic Products (Jabs)
Delving further into the European Medicines Agency documents on Endotoxin, I came across a conference presentation on quality control of Bio Therapeutic Products (BTPs includes Jabs) and found that an expert in Endotoxin harms1 who worked on possible antidotes to Endotoxemia, wants no limit imposed on the amount in Jabs.
Picture credit from an article showing a majority of people in Germany supported Jab Mandates in 2021.2
The German Federal Institute for Drugs and Medical Devices played a key role in oversight of Covid19 Jabs and is supposed to be ensuring quality products by participating in European and wider International regulation of BTPs.
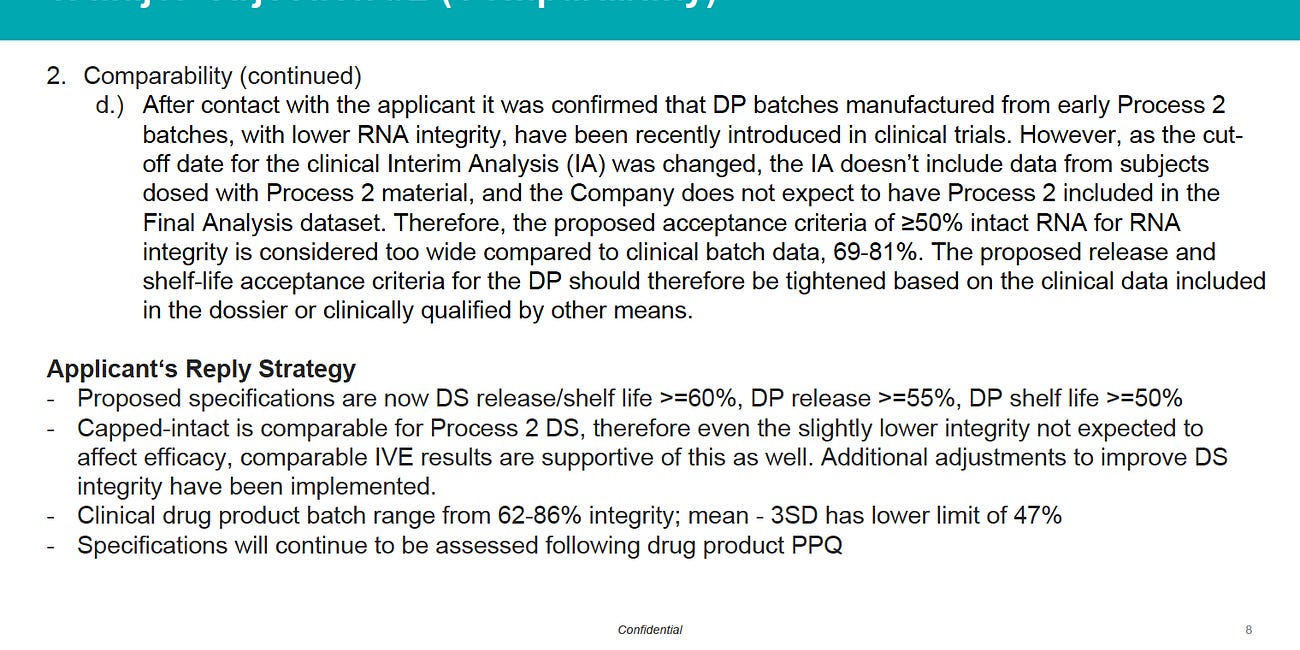
But look at the advice below, basically saying that Quality Control Monographs for BTPs “should NOT INCLUDE LIMITs for parameters that are highly depended (dependent) on the manufacturing process”.
Recall that BioNTech Pfizer lied to the FDA, EMA and other regulatory authorities by claiming the Endotoxin content was controlled, and later made it clear they have absolutely no control in their E. coli vats of Process 2 Poojabs.3
This slide is taken from Dr Brigitte Brake talk in Tallin Estonia in 2016.4
At the time and until April 2023, she was Head of Unit Pharmaceutical Biotechnology, Biologics, Inspections, BfArM Federal Institute for Drugs and Medical Devices Germany. She is now an advisor to the EMA.
Here she was in May 2020 in conference with an interesting collection of people.5
Now we have a clear explanation for why the Paul Ehrlich Institute is not interested in answering questions about Batch to Batch variability of things like residual DNA or Endotoxin.6 They are part of Brigitte Brake’s network.
In my delving, I found that the Paul Ehrlich Institute is actively developing Bacterial Adjuvants for use in Jabs, and so has a massive conflict of interest as a participant in Batch Release. More about that later.
BRIGITTE BRAKE, CLARA LARCHER, THOMAS F. SCHULZ, WOLFGANG PRODINGER, and MANFRED P. DIERICH. 1992. Species Specific Monoclonal Antibodies to Bacteroides fragilis Lipopolysaccharide Protect Mice from Severe Infection.
Deutsches Aerzteblatt International. 2022. https://medicalxpress.com/news/2022-08-attitudes-mandatory-covid-vaccination-germany.html
Production of the Pfizer BioNTech mRNA jabs
It seems that Fifth Column operatives want to hide some of the facts about the massive taxpayer expenditure on the failed Covid19 jabs. Here I will concentrate on Pfizer BioNTech that has achieved market dominance. The production processes for materials used in the Phase 1 trial were not the same as those used in the Phase 2/3 trials, and most likely con…
Brigitte Brake. 2016. Setting Pharmacopoeial Standards for Biotherapeutic Products – An Assessor’s Perspective. EDQM Workshop: Setting Pharmacopoeial Standards for Biotherapeutic Products, 27./28. Sept. 2016, Tallin, Estonia
https://ondemand.casss.org/b/sp/brigitte-brake-408
Pfizer Employees in Australia - Coerced Jabbees, Suffered like the rest
Following a recent Senate hearing in Australia, there has been considerable interest in the fact that Pfizer imported Lots and Batches for exclusive use in its employees and their immediate families. Suggestions that these Lots, which the TGA did not test, were in some way less damaging are False.







What a great plan by the anti-humanists, we can't meet the specification so let's just not have one.
Low bioburden, sterility and endotoxin levels are in direct conflict with producing biologicals.
But they see it as an obstacle in their path rather than a red flag meaning we shouldn't do it.
They have lost their moral compass maybe they will start blaming that on the magnetic field shifting?
What could possibly go wrong