Infanrix Deaths and Injuries related to the ingredients
Sudden Infant Deaths after the GMO Infanrix-Hexa Jab given to Babies are coming under scrutiny. Let's look at what we know about the Supertoxins in the vials
Confusing GSK Brand Names
Infanrix
The US CDC website does not return a result for Infanrix-Hexa, but does for plain old Infanrix:
Each 0.5-mL dose of Infanrix® (GlaxoSmithKline) contains 25 Lf of diphtheria toxoid, 10 Lf of tetanus toxoid, 25 µg of inactivated PT, 25 µg of FHA, and 8 µg of pertactin (69 kiloDalton outer membrane protein). Each 0.5-mL dose contains aluminum hydroxide as adjuvant (not more than 0.625 mg aluminum by assay) and 4.5 mg of sodium chloride. Each dose also contains ≤100 µg of residual formaldehyde and ≤100 µg of polysorbate 80 (Tween 80).1
Note that CDC measures the deliberately added toxoids in Lf (limit of flocculation).2
Toxoids are bacterial toxins that have been treated with Formaldehyde, a Class 1 carcinogen.3
Limit of Flocculation is defined as the quantity of toxin that flocculates in the shortest time with a unit of antitoxin in the time it takes for floccules to appear.
Infanrix Hexa contents
Australia’s TGA does tell us the contents4 of Infanrix-Hexa.
DTPa-HepB-IPV-Hib — diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine
The vaccine is a combination vaccine and consists of both a 0.5 mL monodose pre-filled syringe and a vial containing a lyophilised pellet.
The vaccine needs to be reconstituted by adding the entire contents of the pre-filled syringe containing the liquid component to the vial containing the lyophilised pellet.
Each 0.5 mL reconstituted dose contains:
≥30 IU Diphtheria toxoid1
≥40 IU Tetanus toxoid1
25 µg Pertussis toxoid (PT)1
25 µg Filamentous Haemagglutinin (FHA)1
8 µg Pertactin (PRN)1
10 µg Hepatitis B surface antigen (HBsAg)2,3
40 D-antigen units Inactivated Poliovirus Type 1 (Mahoney)4
8 D-antigen units Inactivated Poliovirus Type 2 (MEF-1)4
32 D-antigen units Inactivated Poliovirus Type 3 (Saukett)4
10 µg Haemophilus influenzae type b polysaccharide (Polyribosylribitol Phosphate)3
20-40 µg Haemophilus influenzae type b polysaccharide (conjugated to tetanus toxoid protein)
1 adsorbed onto aluminium hydroxide/phosphate
2 produced in yeast (Saccharomyces cerevisiae) cells by recombinant DNA technology
3 adsorbed on aluminium phosphate
4 propagated in VERO cells
Also contains traces of:
polymyxin B sulfate
neomycin sulfate
Lactose
Notice that TGA uses a different unit for the toxoids, IU.
Endotoxin and Exotoxin Content ?
The presence of Polymyxin B Sulfate suggests some feable attempt has been made to capture an unknown amount of Endotoxin or Exotoxin.
Haemophilus type b polysaccharide known to be contaminated with Endotoxin
Buried in a 2013 EMA report on a related Sanofi product5 we find the following casual remark on Endotoxin:
The Haemophilus polysaccharide conjugate drug substance (PRP-T) is a polysaccharide prepared from Haemophilus influenzae type b, covalently bound after chemical activation to a carrier (tetanus) protein. These two components are produced, extracted and purified separately using their own seed lot systems and manufacturing processes.
PRP-T production is divided into three main production steps: (1) production of the Haemophilus type b polysaccharide, (2) production of the tetanus protein and (3) conjugation of the Haemophilus type b polysaccharide with the concentrated tetanus protein.
The polysaccharide is precipitated from a culture of H. influenzae type b, purified and subsequently activated (PRP-AH) through chemical linkage/activation.
The tetanus protein is prepared by fermentation of C. tetani (Harvard strain 49205) and lysis, purification and inactivation of the toxin.
The activated polysaccharide is subsequently covalently bound to the tetanus protein. The conjugate product is purified and diluted resulting in the PRP-T drug substance.
For storage, the Haemophilus polysaccharide conjugate concentrated bulk is filled in polypropylene flasks.
The production of the PRP-T drug substance is based on two seed lot systems: (1) Pre-Master, Master and Working Seed Lots for H. influenzae type b; and (2) Master and Working Seed Lots for C. tetani; control of both seed lot systems is acceptable.
The materials used during the production of PRP-T are tested according to either Ph. Eur. or internal specifications (tests and acceptance criteria). Ruminant raw materials used include bovine milk, bovine heart, porcine skin and pancreas, horse blood, poultry feathers and comply with the TSE guidance.
The manufacturing process of the purified Haemophilus type b polysaccharide (PRP) includes an optional reprocessing step, which is performed only once depending on upcoming high endotoxin and pyrogen levels.
Filamentous Haemagglutinin (FHA) loaded with Endotoxin
A recent paper6 stated:
endotoxin in FHA, which in some cases can exceed 40,000 EU/mL acts as a confounding factor when comparing antigen immunogenicity and efficacy (52). Studies determining whether vaccination with MCD-SpyVLP is superior or equivalent to vaccination with FHA would need to be performed with endotoxin-free FHA, which is currently unavailable and technically challenging to produce.
The reference 52 is to Romero et al who measured Endotoxin and suggested that FHA might also contain toxic lipoprotein contaminant.7
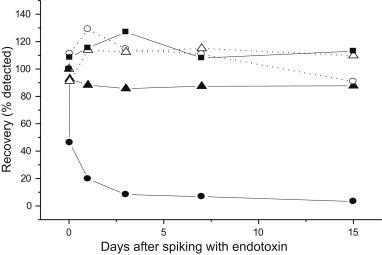
Aluminium masks the Endotoxin
In 2012 scientists in Japan worked out that Aluminium was masking the Endotoxin content of DTaP Jabs that still reduced the body weight of rodents.8
Curious TGA Infanrix Recall in 2012
In 2012 Australia’s TGA announced a recall of 6 batches released in Australia between August 2011 and January 2012 of GSK Infanrix due to Bacterial Contamination9 in the factory:
a surface in the area where one of the manufacturing steps for the vaccine takes place was found to have a small amount of contamination with the bacterium Bacillus cereus, an organism commonly found in food and soil. No contamination of the ingredients or in the vaccine itself was found.
Will the TGA come clean?
Maryanne Demasi is demanding, but not getting, answers via her Freedom Of Information requests.10
https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html
https://www.sciencedirect.com/topics/immunology-and-microbiology/diphtheria-toxoid
https://en.wikipedia.org/wiki/Toxoid
https://immunisationhandbook.health.gov.au/vaccines/infanrix-hexa
Assessment report. Hexacima. Common name: Diphtheria, tetanus, pertussis (acellular, component), hepatitis B (rDNA), poliomyelitis (inactivated) and Haemophilus influenzae type B conjugate vaccine (adsorbed)5 March 2013 EMA/373868/2013 Committee for Medicinal Products for Human Use (CHMP)
Gage M. Pyles, Annalisa B. Huckaby, Maria de la Paz Gutierrez, William T. Witt, Margalida Mateu-Borrás, Spencer R. Dublin, Carleena Rocuskie-Marker, Bethany N. Sesti, Kerrington Peasak, Graham J. Bitzer, Nathaniel Rader, Kelly L. Weaver, Dylan T. Boehm, Nicholas Fitzgerald, Joshua Chapman, Samuel Ulicny, F. Heath Damron, Mariette Barbier. 2024. Virus-like particles displaying the mature C-terminal domain of filamentous hemagglutinin are immunogenic and protective against Bordetella pertussis respiratory infection in mice. https://journals.asm.org/doi/10.1128/iai.00270-24
Villarino Romero R, Hasan S, Faé K, Holubova J, Geurtsen J, Schwarzer M, Wiertsema S, Osicka R, Poolman J, Sebo P. 2016. Bordetella pertussis filamentous hemagglutinin itself does not trigger anti-inflammatory interleukin-10 production by human dendritic cells. Int J Med Microbiol 306:38–47
Michiyo Kataoka, Masaki Ochiai, Akihiko Yamamoto, Yoshinobu Horiuchi. 2012. A need for careful evaluation of endotoxin contents in acellular pertussis-based combination vaccines. https://www.sciencedirect.com/science/article/abs/pii/S1045105611001977
https://www.tga.gov.au/infanrix-hexa-vaccine