FDA finally Bans Supertoxin Red Dye No. 3
If the molecular structures of Food Additives were displayed on packaging, more people might take notice. I is for Iodine. Brief pointer to decades of evidence.
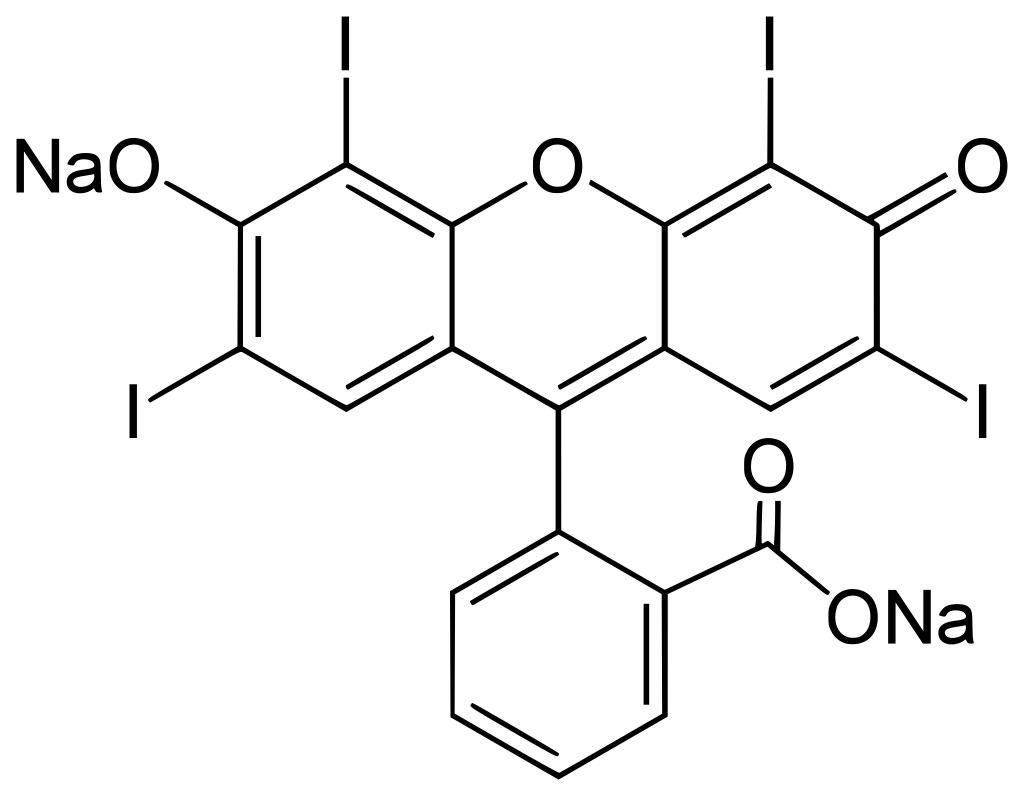
Here it is:
Sodium salt of IUPAC name 2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid.1
Synonyms
Spiro(isobenzofuran-1(3H),9'-(9H)xanthen)-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt
Erythrosine and as a food additive, it has the E number E127.
FDA announcement2 on 15 January 2015. Here is part of it.
The FDA is revoking the authorization for the use of FD&C Red No. 3 as a matter of law, based on the Delaney Clause of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The FDA is amending its color additive regulations to no longer allow for the use of FD&C Red No. 3 in food and ingested drugs in response to a 2022 color additive petition. The petition requested the agency review whether the Delaney Clause applied and cited, among other data and information, two studies that showed cancer in laboratory male rats exposed to high levels of FD&C Red No. 3 due to a rat specific hormonal mechanism.
The way that FD&C Red No. 3 causes cancer in male rats does not occur in humans.
Relevant exposure levels to FD&C Red No. 3 for humans are typically much lower than those that cause the effects shown in male rats. Studies in other animals and in humans did not show these effects; claims that the use of FD&C Red No. 3 in food and in ingested drugs puts people at risk are not supported by the available scientific information.
The Delaney Clause, enacted in 1960 as part of the Color Additives Amendment to the FD&C Act, prohibits FDA authorization of a food additive or color additive if it has been found to induce cancer in humans or animals. This is not the first time the agency revoked an authorization based on the Delaney Clause. For example, in 2018, the FDA revoked the authorization for certain synthetic flavors based on the Delaney Clause in response to a food additive petition.
Manufacturers who use FD&C Red No. 3 in food and ingested drugs will have until January 15, 2027 or January 18, 2028, respectively, to reformulate their products. Other countries still currently allow for certain uses of FD&C Red No. 3 (called erythrosine in other countries). However, foods imported to the U.S. must comply with U.S. requirements.
What an absolute disgrace! Giving Big Pharma an extra year to reformulate!
A useful 1988 review3 of Erythrosine highlights a few key findings over the decades of research.
it produces abnormal telophases in Ehrlich ascites tumour cells and in root tips of Vicia faba (Landa et al., 1965).
It increases the number of micronuclei in rat erythrocytes and chromosomal abnormalities in rat lymphocytes and CHL cells (Godbole and Vaidya, 1980; Ishidate et al., 1984). The dye does not induce gene conversion or 'petite' mutations in yeast (Nagai, 1976; Sankaranarayanan and Murthy, 1979) though, it causes reverse mutations and mitotic gene conversions in S. cerevisiae D7 and XV 185-14C ( Matula and Downie, 1984).
These observations and those reviewed by Combes and Havel and Smith (1982), suggest that erythrosine does interact with the process of mutagenesis.
Genes Affected
The US Government Comparative Toxicogenomics Database shows the Genes affected by this toxin.4 Please click images to enlarge.
Diseases linked to Erythrosine or its descendants
CTD has done considerable Human Curation, marked in blue.
The top 50 of 437 line entries are dominated by cancer studies with hundreds of links to peer reviewed papers.5
Let’s hope Robert F Kennedy Jr. cleans out the FDA with mass sackings.
See also an update with many references from PFPC.6
https://en.wikipedia.org/wiki/Erythrosine
https://www.fda.gov/food/hfp-constituent-updates/fda-revoke-authorization-use-red-no-3-food-and-ingested-drugs
A A Lakdawalla, M S Netrawali. 1988. Mutagenicity, comutagenicity, and antimutagenicity of erythrosine (FD and C red 3), a food dye, in the Ames/Salmonella assay. https://www.sciencedirect.com/science/article/abs/pii/0165121888900833
https://ctdbase.org/detail.go?type=chem&acc=D004923
https://ctdbase.org/detail.go?type=chem&acc=D004923&view=disease