Australians among Humans used in Failed Janssen Sanofi Jab Trial. Shareholders won't be happy !
A multinational Clinical Trial has been halted and Sanofi will write off $250,000,000 for its collaboration as Escherichia coli Jab found to "lack efficacy"
Amazed to look at the 371 locations that recruited elderly people from June 2021 with a recent history of a Invasive extraintestinal pathogenic Escherichia coli disease (IED) urinary tract infection (UTI), or Acute Bacterial Prostatitis (ABP) across 5 continents.
Note the google mapping function displayed on the clincial trial website1 tended not to be accurate.
In Australia the following sites and profiteering companies were used. Note that as of 14 February 2025, many sites were still recruiting, some had completed, but 102 sites were suspended. Looks like bad news travels slowly. I will revisit the site next week.
Please click to enlarge.


Other countries where unsuspecting people aged 60+ were recruited were:
Canada (including the Endotoxin famous University of Guelph)
China
Columbia
Czechia
Denmark
France
Germany
India
Israel
Italy
Japan
Korea
Netherlands
New Zealand
Spain
Sweden
Taiwan
Thailand
United Kingdom
USA
Very interesting that Saudi Arabia is listed as a REMOVED LOCATION COUNTRY - will Donald Trump ask why when he visits to discuss the Ukraine Russia War ?
The Study numbers used in various countries were:
CR109000
2020-005273-27 ( EudraCT Number )
VAC52416BAC3001 ( Other Identifier ) (OTHER: Janssen Research & Development, LLC)
2023-506589-30 ( Registry Identifier ) (REGISTRY: EUCT Number)
2023-506589-30-00 ( Registry Identifier ) (REGISTRY: EUCT number)
NCT04899336
Actual recruitment numbers are not shown.
Exclusion Criteria:
Participant has end-stage renal disease for which dialysis is required
Participant has a contraindication to intramuscular (IM) injections and blood draws example, due to bleeding disorders or a history of difficult blood draws
Participant has a history of acute polyneuropathy (for example, Guillain-Barre syndrome) or chronic inflammatory demyelinating polyneuropathy
Participant has received any Escherichia coli (E. coli) or extraintestinal pathogenic Escherichia coli (ExPEC) vaccine
The Jab
No trial results or publications are listed by the US Government.
ExPEC9V (JNJ-78901563, primary compound number: VAC52416) was a 9-valent intramuscular vaccine candidate. The Placebo is not specified at the NCT04899336 trial site, so I went hunting for more details.
Found a related paper from Janssen researchers in Belgium, Switzerland, The Netherlands and USA.2 But interestingly this refers to a 10-Valent jab.
This phase 1/2a study (NCT03819049) assessed the safety, reactogenicity, and immunogenicity of ExPEC10V (VAC52416) to prevent invasive E coli disease in elderly adults.
This study also used 4-valent ExPEC vaccine (ExPEC4V) and 13-valent Pneumococcal Conjugate Vaccine (PCV13) with no mention of Placebo.
A study evaluating cohort 2 used a double-blind, placebo-controlled design and will be reported separately.
The Placebo ExPEC10V (VAC52416) study was published in Nature.3
Not exactly an unbiased study:
C.A.F. is an employee of Johnson County Clin-Trials, which has performed contracted research for Janssen Research & Development, LLC. M.S., B.S., O.G., T.A.Day, T.A.Davies, G.v.d.D., J.P., and W.H. are employees of Janssen. D.A. was an employee of Janssen at the time of the analysis.
And we see the explanation for dropping one antigen:
Of note, the MOPA immunogenicity analysis of Cohort 1 showed that the O8 strain used in the assay for clinical testing was not able to discriminate a vaccine-induced immune response at Day 15 linked to high baseline titers, necessitating additional assay optimization.
Therefore, a reformulated 9-valent serotype vaccine, ExPEC9V, excluding O8, was advanced for further clinical development in a phase 3 study (E.mbrace, NCT04899336) that may provide valuable data regarding the efficacy of ExPEC9V for the prevention of IED.
The nine specific types (serotypes) of E. coli bacteria used were: O1, O2, O4, O6, O15, O16, O18, O25, and O75.
Efficacy was clearly not acceptable as the world was told by Jab pushing Reuters.4
A Study of Vaccination With 9-valent Extraintestinal Pathogenic Escherichia Coli Vaccine (ExPEC9V) in the Prevention of Invasive Extraintestinal Pathogenic Escherichia Coli Disease in Adults Aged 60 Years And Older With a History of Urinary Tract Infection in the Past 2 Years. NCT04899336. Janssen Research & Development, LLC. https://clinicaltrials.gov/study/NCT04899336
Carlos A Fierro, Michal Sarnecki, Joachim Doua, Bart Spiessens, Oscar Go, Todd A Davies, Germie van den Dobbelsteen, Jan Poolman, Darren Abbanat, Wouter Haazen. 2023. Safety, Reactogenicity, Immunogenicity, and Dose Selection of 10-Valent Extraintestinal Pathogenic Escherichia coli Bioconjugate Vaccine (VAC52416) in Adults Aged 60-85 Years in a Randomized, Multicenter, Interventional, First-in-Human, Phase 1/2a Study. https://academic.oup.com/ofid/article/10/8/ofad417/7241484
Carlos A. Fierro, Michal Sarnecki, Bart Spiessens, Oscar Go, Tracey A. Day, Todd A. Davies, Germie van den Dobbelsteen, Jan Poolman, Darren Abbanat and Wouter Haazen. A randomized phase 1/2a trial of ExPEC10V vaccine in adults with a history of UTI. https://www.nature.com/articles/s41541-024-00885-1
J&J, Sanofi stop E.coli vaccine trial due to low effectiveness. https://www.reuters.com/business/healthcare-pharmaceuticals/jj-discontinue-ecoli-vaccine-candidate-study-2025-02-13/



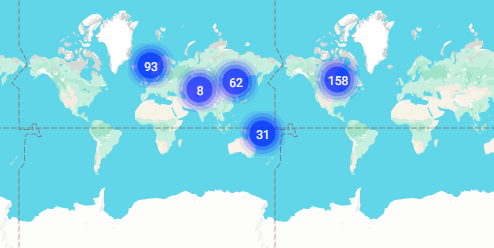
'Low effectiveness'? What could that be interpreted to mean? Surely with the enormous size of the trial they would have had some vague idea of effectiveness. Or did far too many people die eventually in some earlier unpublished trials to make proceeding unconscionable? The mind boggles.